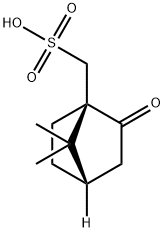
(1R)-(-)-10-Camphorsulfonic acid: Synthesis, application, pharmacokinetics and toxicity
Apr 11,2023
General description
(1R)-(-)-10-Camphorsulfonic acid is prepared from camphor by sulfonation reaction. It can be used as medicine intermediate and amino-acid drug resolution agent. It is veterinary central stimulant, can also be used as a respiratory stimulant. Optically active R- and S-Camphorsulfonic acid are important chiral isomers for drug resolution. At present, only (1R)-(-)-10-Camphorsulfonic acid can be obtained from natural camphor by bromination and sulfonation. Its appearance is as follows:

Figure 1 Appearance of (1R)-(-)-10-Camphorsulfonic acid.
Chemical synthesis
(1R)-(-)-10-Camphorsulfonic acid can be synthesized by single step according to the previous work [1]. To a suspension 2-decanol (158 mg, 1 mmol) (and decane (an internal standard for the determination of GC yield) in t-butyl alcohol (3 mL) was added 30% aq H2O2 (680 μL, 6 mmol), and the resulting suspension was stirred at 80 °C for 24 h. After the reaction mixture was cooled to 25 °C, it was filtered with EtOAc (20 mL) through a membrane filter (The catalyst was recovered onto the membrane filter.), and the filtrate was subjected to GC analysis for the determination of GC yield. The filtrate was washed with sat. Na2S2O3, dried over MgSO4, dried in vacuo, and purified by silica gel chromatography. (1R)-(-)-10-Camphorsulfonic acid. Yield 87%. 1H NMR (500 MHz, CDCl3): δ 2.37-2.32 (m, 1H), 2.10-2.08 (m, 1H), 1.98-1.91 (m, 1H), 1.86-1.82 (m, 1H), 1.71-1.65 (m, 1H), 1.43-1.31 (m, 2H), 0.96 (s, 3H), 0.91 (s, 3H), 0.83 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 219.72, 57.74, 46.83, 43.34, 43.08, 29.96, 27.10, 19.83, 19.19, 9.31. EI-MS m/z: 153 (M+), 137, 108, 95 (bp), 81, 69, 55.
Application
(1R)-(-)-10-Camphorsulfonic acid is believed to be toxic to insects and is thus sometimes used as a repellent [2]. (1R)-(-)-10-Camphorsulfonic acid is used as an alternative to mothballs. Its crystals are sometimes used to prevent damage to insect collections by other small insects. It is kept in clothes used on special occasions and festivals, and also in cupboard corners as a cockroach repellent. The smoke of its crystal or incense sticks can be used as an environmentally-friendly mosquito repellent. In addition, (1R)-(-)-10-Camphorsulfonic acid is commonly applied as a topical medication as a skin cream or ointment to relieve itching from insect bites, minor skin irritation, or joint pain [3]. It is absorbed in the skin epidermis, where it stimulates nerve endings sensitive to heat and cold, producing a warm sensation when vigorously applied, or a cool sensation when applied gently. The action on nerve endings also induces a slight local analgesia.
Pharmacokinetics
(1R)-(-)-10-Camphorsulfonic acid is well absorbed following oral ingestion. It has been detected in the blood as early as 20 minutes after ingestion. Although the vast majority of reported cases of (1R)-(-)-10-Camphorsulfonic acid toxicity are due to oral ingestion, a few case reports suggest absorption by inhalation and through the skin [4].Nasal instillation has also been implicated in toxicity.19 Following absorption, (1R)-(-)-10-Camphorsulfonic acid undergoes hepatic metabolism and is excreted as pharmacologically inactive glucuronide compounds in the urine.
Toxicity
(1R)-(-)-10-Camphorsulfonic acid produces gastrointestinal and central nervous system (CNS) irritation following overdosage. Following ingestion, nausea and vomiting are usual initial findings. These may be followed by hyperactivity, agitation, restlessness, and headache. Hallucinations and seizures occur in severe overdose. Several authors, however, have commented on the sudden appearance of seizures without prior symptomatology. Hepatotoxicity as evidenced by abnormal liver function tests and fatty infiltration has been reported [4]. There is a case reported of a 6-month-old child who was thought to have Reye's syndrome (antecedent viral upper respiratory tract infection, coma, abnormal liver function tests, and hypoglycemia), but his liver biopsy was non-pathognomonic [5]. He was subsequently discovered to have received 25 g of (1R)-(-)-10-Camphorsulfonic over 5 months in a homemade cold preparation.
Renal abnormalities such as albuminuria and proteinuria have also been reported [6]. Transplacental passage of (1R)-(-)-10-Camphorsulfonic has been reported in several cases. To date, there is one case of fetal death reported that may possibly have been due to (1R)-(-)-10-Camphorsulfonic. However, the mother was also suffering from preeclampsia, and its relationship and contribution to the death cannot be ruled out. There are cases of normal deliveries in which the amniotic fluid and the baby had strong odors of (1R)-(-)-10-Camphorsulfonic after maternal ingestion [7].
References
[1]Yamada et al. H2O2-Oxidation of Alcohols Promoted by Polymeric Phosphotungstate Catalysts. Organic Letters, 2010, 12(20), 4540-4543.
[2]The Housekeeper's Almanac, or, the Young Wife's Oracle! for 1840!. No. 134. New-York: Elton, 1840. Print.
[3]Camphor cream and ointment. Drugs.com. August 25, 2019. Retrieved 19 February 2020.
[4]Skoglund et al. Prolonged seizures due to contact and inhalation exposure to camphor. A case report. Clin. Pediatr. 1977, 16:901-902.
[5]Jimenez et al. Chronic camphor ingestion mimicking Reye's syndrome. Gastroenterology, 1983 84:394-398.
[6]Smith et al. Camphor poisoning. Anatomical and pharmacologic study; report of a fatal case; experimental investigation of protective action of barbiturate. Am. J. Patho!. 1954, 30:857-867.
[7]Riggs et al. Camphorated oil intoxication in pregnancy. Report of a case. Obstet. Gyneco!. 1965, 25:255-258.
- Related articles
- Related Qustion
N,N-Dimethylformamide is a common polar solvent that has a worldwide annual production, and is used in a wide variety of industrial processes.....
Apr 11,2023Organic Synthesis IntermediateMonomethyl auristatin E is an agent with anti-mitotic activity, which is often used to inhibit cell division by blocking the polymerization of tubulin.....
Apr 11,2023Drugs(1R)-(-)-10-Camphorsulfonic acid
35963-20-3You may like
(1R)-(-)-10-Camphorsulfonic acid manufacturers
- (1R)-(-)-10-Camphorsulfonic acid
-

- $10.00 / 1kg
- 2024-05-22
- CAS:35963-20-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20ton
- L(-)-Camphorsulfonic acid
-

- $15.00/ kg
- 2024-04-24
- CAS:35963-20-3
- Min. Order: 1kg
- Purity: 99.912%
- Supply Ability: 10ton
- l-10-camphorsulfonic acid
-

- $0.00 / 1Kg
- 2024-03-29
- CAS:35963-20-3
- Min. Order: 1Kg
- Purity: 99.9%
- Supply Ability: 200tons