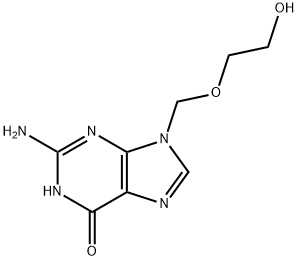
Acyclovir: Antiviral Medication for Herpes with Promising Pharmacokinetics and Potency
Jan 9,2024
General Description
Acyclovir is an antiviral medication used to treat herpes simplex, varicella zoster, and Epstein-Barr virus infections. Its pharmacokinetics involve limited oral absorption with steady-state peak plasma concentrations of 0.5 μg/ml. Intravenous administration results in a mean peak plasma concentration of 9.7 μg/ml, and topical administration yields high aqueous humor concentrations. Acyclovir is detected in various organs and exhibits different concentrations in cerebrospinal fluid, skin vesicles, and saliva. Approximately 15% is metabolized, with the remainder excreted unchanged in urine. The drug has a short elimination half-life but can have a 7-fold increase in patients with end-stage renal impairment. It demonstrates potent activity against herpes simplex viruses, varicella zoster virus, and Epstein-Barr virus. Recommended dosages vary by indication and route of administration, with specific guidelines for different conditions and patient populations.

Figure 1. Tablets of acyclovir
Pharmacokinetic
Acyclovir is an antiviral medication used to treat herpes simplex types I and 2, varicella zoster, and Epstein-Barr virus infections. Intravenous administration of acyclovir at a dose of 5 mg/kg every 8 hours results in a steady-state mean peak plasma concentration of 9.7 μg/ml. Oral absorption of acyclovir is limited, with a bioavailability ranging from 15 to 30%. Steady-state mean peak plasma concentrations with oral administration of the recommended dose of 200mg every 4 hours were approximately 0.5 μg/ml. Topical administration of acyclovir results in limited systemic absorption but relatively high concentrations in the aqueous humor with multidose ocular application of the 3% ointment every 5 hours. Following intravenous therapy, acyclovir was detected in various organs, including the kidney, lung, nervous tissue, liver, and heart. Cerebrospinal fluid and skin vesicle concentrations following intravenous therapy, and saliva concentrations following oral therapy were approximately 50%, 100%, and 13% of simultaneous plasma concentrations, respectively. Approximately 15% of a dose of acyclovir is metabolized to an inactive metabolite, and the remainder is excreted unchanged in the urine. The elimination half-life in patients with normal renal function is 2 to 3 hours, while it is increased 7-fold in patients with end-stage renal impairment. The disposition of acyclovir in children is similar to that in adults, but neonates have decreased total body clearance and slightly increased elimination half-life. 1
Antiviral activity
Acyclovir is an antiviral drug that exhibits strong activity against various strains of herpes simplex viruses (HSV) 1 and 2, varicella zoster virus (VZV), Epstein-Barr virus (EBV), and, to a limited extent, cytomegalovirus (CMV). In vitro studies have shown that acyclovir is more effective against HSV-1 than idoxuridine, trifluridine, or vidarabine, and it has similar potency to cytarabine. Against VZV, the comparative potency of acyclovir varies among studies. Other antivirals like idoxuridine, trifluridine, and vidarabine demonstrate greater activity against CMV than acyclovir. In animal models, acyclovir has demonstrated efficacy in treating ocular, cutaneous, genital, encephalitic, and neonatal HSV infections. The effectiveness of acyclovir in these models depends on dosage, route, and timing of administration. Comparatively, acyclovir is as effective or more effective than other nucleoside analogue drugs. Acyclovir can suppress the reactivation of latent HSV in neural ganglia but does not eliminate established latent virus. Early initiation of topical or systemic therapy can prevent viral latency following primary infections in some cases. However, clinical trials have not consistently replicated this finding. Although resistant strains of HSV-1, HSV-2, and VZV can be isolated in vitro and in animal models, clinically resistant strains are rare. Acyclovir inhibits viral DNA replication by being selectively taken up by infected cells and phosphorylated by viral thymidine kinase. Acyclovir triphosphate, the active form of the drug, specifically targets viral DNA polymerase. Due to its high specificity, acyclovir has a high therapeutic index. 2
Dosage
The recommended dosages of Acyclovir vary depending on the specific indication and route of administration. For herpes simplex keratitis, a 3% acyclovir ophthalmic ointment is applied every 4 hours while awake (5 times daily) for at least 3 days after complete healing. In cases of initial herpes genitalis and non-life-threatening mucocutaneous herpes simplex virus infections in immunocompromised patients, a 5% topical ointment is recommended. A ribbon of ointment measuring approximately 1cm long per 10 cm^2 surface area should be applied as soon as possible after symptom onset. For herpes simplex infections of the skin and mucous membranes, including initial and recurrent genital herpes, the recommended oral dosage is 200mg taken 5 times daily (approximately every 4 hours) for 5 days. In severe initial clinical episodes of genital herpes or mucosal and cutaneous herpes simplex virus infections in immunocompromised patients, intravenous acyclovir therapy is recommended. The dosage is 5 mg/kg infused over 1 hour every 8 hours for 5 days or 7 days. Lower infusion concentrations are preferred to avoid phlebitis or inflammation, and hydration is necessary to prevent drug precipitation in the renal tubules. In children under 12 years, dosing is based on body surface area. Dosage adjustments are required for patients with moderate to severe renal function impairment. 1
Reference
1. Richards DM, Carmine AA, Brogden RN, Heel RC, Speight TM, Avery GS. Acyclovir. A review of its pharmacodynamic properties and therapeutic efficacy. Drugs. 1983 Nov;26(5):378-438.
2. O'Brien JJ, Campoli-Richards DM. Acyclovir. An updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1989 Mar;37(3):233-309.
- Related articles
- Related Qustion
- Acyclovir: Applications and side effects Jun 27, 2023
Acyclovir is an antiviral drug. However, it is not a cure for these infections. The viruses that cause these infections continue to live in the body even between outbreaks.
- The uses and side effects of Acyclovir Oct 11, 2019
Acyclovir is an antiviral drug. However, it is not a cure for these infections. The viruses that cause these infections continue to live in the body even between outbreaks. Acyclovir decreases the severity and length of these outbreaks. It
Cinnamic acid in plant-based foods stimulates insulin secretion, but its limited bioavailability led to new formulations like nanoparticles for improved effectiveness against chronic diseases.....
Jan 9,2024APITetrahydro-4H-pyran-4-one is synthesized using various reagents and solvents, and has pharmaceutical applications as an SSAO inhibitor and a modulator of NMDA receptors.....
Jan 9,2024APIAcyclovir
59277-89-3You may like
- Acyclovir
-

- $0.00 / 1KG
- 2024-05-31
- CAS:59277-89-3
- Min. Order: 0.10000000149011612KG
- Purity: 99% up, High Density
- Supply Ability: 20 tons
- Acyclovir
-
- $0.00 / 1kg
- 2024-05-31
- CAS:59277-89-3
- Min. Order: 1kg
- Purity: 99.0%
- Supply Ability: 10tons
- Acyclovir
-

- $10.00 / 1kg
- 2024-05-30
- CAS:59277-89-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100Tons