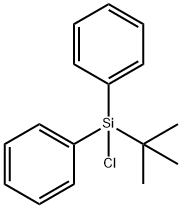
Application of tert-Butylchlorodiphenylsilane
Mar 21,2022
General description
Tert butyl diphenylchlorosilane known as silane protector, is used to replace the active hydrogen in silane based compounds (such as hydrogen in hydroxyl, carboxyl and amino groups) to form stable intermediates; Then, other groups of the intermediate are carrying out some reactions; After the reaction, through the chemical Book hydrolysis reaction, the silane group is removed to regenerate the group originally protected by silyl, and some specific compounds are synthesized. Silane is widely used in organic synthesis, especially in drug synthesis, because of its high conversion rate of protection and deprotection reaction, even quantitative reaction.[1]
Application
Tert-Butylchlorodiphenylsilane is used to synthesize p-benzophenoxazole, a compound with antagonistic activity to thromboxane receptor. It can be used to treat circulatory diseases, angina pectoris and stroke. Tert-Butylchlorodiphenylsilane is used as pharmaceutical intermediates and in organic synthesis A silicon based protective group; Tert-Butylchlorodiphenylsilane is used as pharmaceutical intermediates and in organic synthesis A silicon based protective group; It is used in the synthesis of pharmaceutical intermediates or other polymers. Tert-Butylchlorodiphenylsilane is used for protecting alcohol and preparing silicone ether Silicide for protecting alcohol and preparing silicon ether.[2]
Mechanism of action
Tert butyl diphenylchlorosilane (tbdpscl) is a commonly used silylation reagent. The main purpose of the reagent is to protect alcohol hydroxyl group, phenol hydroxyl group, primary amine group and the preparation of enol silicone ether.The conditions of protective alcohol are basically the same as tbscl. It is generally carried out in DMF, ch2ci2 and THF. Imidazole, triethylamine and NAH are used as basic reagents. Among them, DMF imidazole system has strong reactivity and can be used for the protection of secondary alcohols. The CH2Cl2 triethylamine DMAP system can reliably realize the selective protection of primary alcohols.[3]Tbdps ether of alcohol has good stability to acid, alkali, reducing agent, oxidant, transition metal catalysis, free radical and other conditions. Compared with the corresponding TBS derivatives, their stability to acid is about 100 times higher, and their tendency to migrate to adjacent hydroxyl groups is small. Therefore, tbdps ether can be selectively retained and highly selectively removed under acidic conditions (formula 3). However, alkyl tbdps ether is sensitive to some strong alkaline reagents, such as NaOH (5 mol / L) / EtOH, nah / hmpa-h2o, etc. , while TBS ether is relatively stable.[4]
The protection of phenolic hydroxyl group can refer to the reaction conditions of alcohol, and the product is stable to Wittig reaction and Grignard reaction. Due to the conjugation of lone pair electrons of oxygen with aryl, the Lewis basicity of aryl tbdps ether is weaker than that of alkyl analogues. Therefore, tbdps protection of selective removal of alcohol light groups by acidic reagents is generally used. On the contrary, phenoxy anion is a better leaving group, and the selective removal of phenolic hydroxyl tbdps ether protective group can be realized by using basic reagent[5]
Synthesis
Add 200ml of tetrahydrofuran, 12g of magnesium and 6G of chloropropene (the remaining 40g of chloropropene is added into the dropping funnel) to a 500ml reactor with snake condenser, electric stirrer, dropping funnel, thermometer and heater. Under stirring, heat slowly to initiate the reaction and control the reaction temperature of 50 ~ 75 ℃. Once the reaction is initiated, stop heating immediately. If the reaction is violent, Quickly cool down the reactor. After the reaction is stable, open the dropping funnel, drop chloropropene into the reactor, drop it to room temperature, then add 0.2g sodium thiocyanate chemicalbook and 0.2g cuprous chloride catalyst into the reactor, add 127g Diphenyldichlorosilane into the dropping funnel, drop Diphenyldichlorosilane into the reactor under stirring, and the dropping is completed, The materials in the reactor are heated to 90 ~ 150 ℃ and reacted at constant temperature for 4 ~ 7 hours. After the reaction is completed and reduced to room temperature, 100ml of inert solvent toluene is added into the reactor, stirred evenly, the generated solution is filtered to remove solid salt, the filtrate is distilled, the flux is evaporated, and then vacuum distillation is carried out. The fraction at 200 ℃ is collected at 0.09kpa to obtain the product tert butyl diphenylchlorosilane with a content of 98%.[6]
Figure 1 the synthesis route of tert-Butylchlorodiphenylsilane
References
1(a) Wuts,P. G. M.; Greene, T. W. Greene's Protective Groups in Organic Synthesis; 4th ed.,John Wiley & Sons:Hoboken, NJ,2007. (b) Nelson,T. D.; Crouch, R. D. Synthesis 1996,1031. (c) Crouch, R. D. Tetrahedron 2004, 60,5833.
2. (a)Hanessian, S.; Lavallee, P. Can. J. Chem.1975, 53, 2975. (b) Nicolaou, K. C.; Papahatjis, D. P.;Claremon, D. A.; Magolda, R. L.; Dolie, R. E. J.Org. Chem. 1985, 50, 1440.
3. Roche, C.; Desroy, N.; Haddad, M.; Phansavath, P.; Genet, J.-P. Org.Lett, 2008,10, 3911.
4.Pattenden, G.; Ashweek, N.J.; Baker-Glenn, C. A. G.; Kempson, J.; Walker, G. M.; Yee, J. G. K.Org. Biomol. Chem. 2008, 6, 1478.
5. (a)Loh,T.-P.;Feng’L.-C.Tetrahedron Lett.2001,42,3223. (b) Shekhani,M.S.;Khan,K.M.;Mahmood, K.; Shah, P. M.; Malik, S. Tetrahedron Lett. 1990, 31,1669.
6. Lee, A. S.-Y.; Yeh, H.-C.; Shie, J.-J. Tetrahedron Lett. 1998, 39, 5249.
- Related articles
- Related Qustion
Glyphosate is a biocide herbicide developed by the famous American company Monsanto.....
Mar 21,2022APIEthylenediaminetetraacetic acid disodium salt also known as disodium ethylenediamine tetraacetate, commonly known as disodium ethylenedicarboxylate....
Mar 21,2022Organic Synthesis Intermediatetert-Butylchlorodiphenylsilane
58479-61-1You may like
tert-Butylchlorodiphenylsilane manufacturers
- tert-Butylchlorodiphenylsilane
-

- $20.00 / 1g
- 2024-06-03
- CAS:58479-61-1
- Min. Order: 10g
- Purity: 98%
- Supply Ability: 2000kg
- tert-Butyldiphenylchlorosilane
-

- $0.00 / 1kg
- 2024-06-03
- CAS:58479-61-1
- Min. Order: 0.1kg
- Purity: 98%
- Supply Ability: 50tons
- tert-Butylchlorodiphenylsilane
-

- $70.00 / 1kg
- 2024-05-10
- CAS:58479-61-1
- Min. Order: 10kg
- Purity: 0.99
- Supply Ability: 20tons