Benzophenone imine: applications as an ammonia equivalent
Aug 30,2023
General Description
Benzophenone imine and its derivatives are useful ammonia equivalents for synthesizing primary amines, readily achieving the selective formation of primary amines and obtaining easily deprotectable imines as the product. In recent years, there has been increasing interest in using phenol derivatives as alternative pseudo-halides in cross-coupling reactions with aryl (pseudo-)halides. Additionally, palladium-catalyzed cross-coupling reactions between alkyl bromides and benzophenone imine have been explored. Further research revealed higher selectivity for N-alkylation with electron-deficient imines. Another method for synthesizing chiral α-amino acids is through transition metal-catalyzed enantioselective insertion of carbenes into N-H bonds. This method has overcome limitations of previous reports by optimizing reaction conditions with specific catalysts.

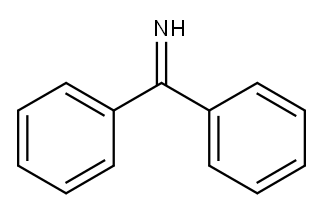
Figure 1. Benzophenone imine
Applications as an ammonia equivalent
Cross-coupling reactions with aryl (pseudo-)halides
The conventional Buchwald-Hartwig amination method typically utilizes aryl halides or triflates as starting materials. However, there has been growing interest in using phenol derivatives as alternative pseudo-halides in recent years. This approach requires high activation energy to cleave stable Csp2O bonds. To overcome this challenge, electron-rich nickel catalysts have been employed. Although these catalysts enable the synthesis of secondary or tertiary amines, accessing primary amines has remained difficult. In a study by Rueping and colleagues, a nickel-catalyzed Buchwald-Hartwig-type amination was developed using benzophenone imine via CO bond cleavage. By optimizing the reaction conditions and utilizing pivalate as a leaving group, it was found that the combination of Ni(cod)2 and a dcype ligand yielded the best results. This methodology proved effective for pivalate, carbonates, carbamates, and sulfamates, while more stable methoxy and ethoxy groups did not yield the desired products. The resulting imine products could be transformed into primary or secondary amines through hydrolysis or reduction, respectively. 1
Cross-coupling reactions with alkyl bromides
In 2016, Hartwig and his team conducted groundbreaking research on palladium-catalyzed cross-coupling reactions between secondary and tertiary alkyl bromides and benzophenone imine. They explored various phosphine ligands and identified tBuCy2P, a bulky trialkylphosphine ligand, which resulted in the highest yield of N-alkyl imines. To simplify the process, they selected a pre-complexed catalyst, (t-BuCy2P)2Pd(H)Br, as the optimal catalyst. Subsequent investigations revealed that electron-deficient imines exhibited higher selectivity for the desired N-alkylation. The starting N-unsubstituted 3,3'-bis(trifluoromethyl)benzophenone imine underwent hydrolysis on silica gel, but the N-alkyl imine products were stable on silica gel, making it easier to isolate the products. Mechanistically, both exo- and endo-2-bromonorbornane yielded the product predominantly in the exo form with over 97% yield. When enantioenriched alkyl bromide was used, the product obtained after deprotection and reprotection of the imine moiety was a complete racemate. These results indicate that the reactions proceed through radical intermediates, which was further supported by radical clock experiments. 2
Catalytic N–H insertion of carbenes
Transition metal-catalyzed enantioselective insertion of α-diazocarbonyl compounds into N-H bonds is an efficient method for synthesizing chiral α-amino acids. However, limitations regarding substrate scope, enantioselectivity, and protective group removal have persisted in previous reports. To address these challenges, Liu and his team developed a method for enantioselective insertion of carbenes into the N-H bond of benzophenone imine. Building upon their previous work on inserting carbenes into the O-H bond of carboxylic acids, they optimized the reaction conditions using Rh2(esp)2 and an L-Ramipril-derived chiral guanidine catalyst. This resulted in high yields and enantioselectivity, with even better results achieved using 4,4'-difluorobenzophenone imine. The substrate scope was expanded, and with slight modifications to the reaction conditions, both aryl- and alkyl-substituted α-diazo esters produced the desired product with high yields and good to high enantioselectivity, even in the presence of various functional groups. However, in some cases, side reactions led to lower to moderate yields. The reactions were scalable, and deprotection of the benzophenone imine yielded unprotected α-amino acids without any loss of enantiopurity. Interestingly, the absolute stereochemistry of the product derived from aryl-substituted α-diazo esters was opposite to that derived from alkyl-substituted ones. 3
Reference
1. Yue H, Guo L, Liu X, Rueping M. Nickel-Catalyzed Synthesis of Primary Aryl and Heteroaryl Amines via C-O Bond Cleavage. Org Lett, 2017, 19(7):1788-1791.
2. Peacock DM, Roos CB, Hartwig JF. Palladium-Catalyzed Cross Coupling of Secondary and Tertiary Alkyl Bromides with a Nitrogen Nucleophile. ACS Cent Sci, 2016, 2(9):647-652.
3. Ford A, Miel H, Ring A, Slattery CN, Maguire AR, McKervey MA. Modern Organic Synthesis with α-Diazocarbonyl Compounds. Chem Rev, 2015, 115(18):9981-10080.
- Related articles
- Related Qustion
- Benzophenone Imine: Chemical Properties, Applications in Medical Diagnostics and its Synthesis Method Apr 28, 2024
Benzophenone Imine's reactivity aids in complex structure creation. Crucial for brain tumor PET imaging, it's synthesized efficiently via ruthenium-catalysis.
- Benzophenone Imine: Versatile Compound for Synthesizing Unnatural α-Amino Acids Jan 4, 2024
Benzophenone imine is a clear liquid with unique properties and versatile synthetic applications, requires careful handling due to its potential for causing skin, eye, and respiratory irritation.
- Applications of Benzophenone imine Nov 22, 2019
Benzophenone imine(Ph2CH=NH) is an organic compound widely used as a reagent for the protection of primary amines, and the starting materials to synthesize aniline.
2-Methoxy-4-nitroaniline is a versatile compound used in industries for dyes, pharmaceuticals, and specialty chemicals. It has vibrant colors and participates in diverse reactions.....
Aug 30,2023APIPyridinium p-Toluenesulfonate is a white crystalline powder with versatile properties. It acts as a mild acid catalyst, facilitating various reactions. Safety precautions should be followed.....
Aug 31,2023APIBenzophenone imine
1013-88-3You may like
Benzophenone imine manufacturers
- Benzophenone imine
-

- $30.00 / 1kg
- 2023-11-21
- CAS:1013-88-3
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 10 tons
- Benzophenone imine
-

- $0.00 / 1KG
- 2023-09-06
- CAS:1013-88-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg
- Benzophenone imine
-

- $15.00 / 1KG
- 2021-07-13
- CAS:1013-88-3
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton