D-(-)-Salicin: Phytochemistry, Biosynthesis and Metabolism
Mar 11,2024
General Description
D-(-)-Salicin, a phenolic glycoside with significant phytochemical importance, exhibits a complex structure characterized by a β-D-glucose moiety and salicyl alcohol linked via a β-1,1′-D-glycosidic bond. Its biosynthesis, though not fully elucidated, involves a series of enzymatic steps starting from L-phenylalanine within the phenylpropanoid pathway, highlighting nature's intricate chemical orchestration. Upon oral ingestion, D-(-)-Salicin metabolizes into salicylic acid through glycon hydrolysis and oxidation, undergoing further transformation in the liver and kidneys to various metabolites, facilitated by the cytochrome P-450 system and conjugation reactions. This metabolic versatility underscores D-(-)-Salicin's therapeutic potential, particularly its anti-inflammatory and pro-apoptotic effects, demonstrating the compound's biochemical significance and utility in medicinal studies.

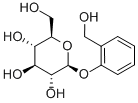
Figure 1. D-(-)-Salicin
Phytochemistry
D-(-)-Salicin, a phenolic glycoside first discovered in nature, has a significant role in phytochemistry due to its unique structure and properties. With a molecular mass of 286.27782 g/mol, its chemical composition includes a β-D-glucose moiety and salicyl alcohol, linked by a β-1,1′-D-glycosidic bond. This compound is notable for its inclusion of five chiral carbon centers, all contributed by the β-D-glucose part, emphasizing its complex stereochemistry. The IUPAC name, reflecting its detailed structure, is (2R,3S,4S,5R,6S)-2-(hydroxymethyl)-6-[2-(hydroxymethyl)phenoxy]oxane-3,4,5-triol, which highlights its multiple hydroxyl groups and hydroxymethyl components. These hydroxyl groups act as hydrogen bond donors, while the seven oxygen atoms present serve as hydrogen bond acceptors, influencing D-(-)-Salicin's solubility and interaction with other molecules. The presence of nine rotational bonds allows for a variety of conformations, affecting its physical and chemical behavior. The polarity of D-(-)-Salicin, attributed to its structural features, necessitates the use of polar solvents like boiling water and ethyl alcohol for its extraction. Moreover, the β-D-glucose moiety not only contributes to the molecule's chiral centers but also enhances its physicochemical properties, making D-(-)-Salicin a compound of interest in various scientific and medicinal studies. 1
Biosynthesis
The biosynthesis of D-(-)-Salicin, despite the lack of detailed elucidation regarding its specific pathway, genes, or enzymes in literature, has been partially uncovered through biotechnological approaches and experiments using leaf tissues and radio-labeled precursors in Salix and Populus species. These studies have shed light on the process, revealing that D-(-)-Salicin's biosynthesis is intricately linked to the phenylpropanoid pathway, beginning with the amino acid L-phenylalanine. This pathway involves a series of five key steps to transform L-phenylalanine into D-(-)-Salicin. The first step is deamination, where the amino group of L-phenylalanine is removed, leading to the formation of a cinnamic acid derivative. Following deamination, ortho-hydroxylation occurs, introducing a hydroxyl group into the aromatic ring, which is crucial for forming the characteristic salicyl alcohol structure. The third step is β-oxidation, a process that typically involves the shortening of fatty acid chains but here likely refers to modifications that further prepare the molecule for subsequent transformations. C2 unit elimination then follows, a step that adjusts the carbon skeleton to more closely resemble the final salicin structure. Finally, glucosylation occurs, attaching a glucose moiety to the salicyl alcohol precursor via a β-glycosidic bond, completing the biosynthesis of D-(-)-Salicin. This pathway highlights the complex interplay of enzymatic reactions required to synthesize D-(-)-Salicin from a simple amino acid, showcasing nature's ability to orchestrate elaborate chemical transformations. 2
Metabolism
The metabolism of D-(-)-Salicin, upon oral ingestion, initiates with its conversion into salicylic acid, a process facilitated by glycon hydrolysis and oxidation in the gastrointestinal tract and bloodstream. This metabolic pathway mirrors that of acetylsalicylic acid, which also metabolizes into salicylic acid through the action of esterases. Salicylic acid then undergoes further transformation in the liver and kidneys, primarily through the cytochrome P-450 enzyme system, resulting in various metabolites like 2,3,5-trihydroxy benzoic acid and catechol. It is also involved in conjugation reactions, forming compounds such as salicyluric acid and salicylacyl glucuronide. These metabolic processes underscore the body's ability to alter D-(-)-Salicin and related compounds, enhancing their clearance and therapeutic efficacy, particularly in anti-inflammatory and pro-apoptotic applications. The carboxylic group of salicylic acid plays a pivotal role in these reactions, showcasing the compound's biochemical versatility. 1
Reference
1. Mahdi JG. Biosynthesis and metabolism of β-d-salicin: A novel molecule that exerts biological function in humans and plants. Biotechnol Rep (Amst). 2014;4:73-79.
2. Zenk MH. Pathways of salicyl alcohol and salicin formation in Salix purpurea L. Phytochemistry. 1967;6:245–252.
- Related articles
- Related Qustion
- D(-)-Salicin:anti-inflammatory, purgative and analgesic agents Sep 15, 2023
D(-)-Salicin, an organic compound with the chemical formula C13H18O7, extracted from white willow bark, is a glycoside compound.
- Inhibition of D(-)-Salicin Oct 14, 2019
D(-)-Salicin is a traditional medicine which has been known to exhibit anti-inflammation and other therapeutic activities, it can inhibit the LPS-induced inflammation in RAW264.7 cells and mouse models.
Capmatinib targets MET exon 14 skipping mutations, inhibiting proliferation and migration in non-small cell lung cancer. It has predictable pharmacokinetics and manageable adverse reactions.....
Oct 31,2024APISynthesizing stannous sulfate from tin waste promotes sustainability and enhances lead-acid batteries and smart sensing devices through improved material properties.....
Oct 29,2024Inorganic saltsD-(-)-Salicin
138-52-3You may like
- Salicin
-

- $29.00 / 1mL
- 2024-10-31
- CAS:138-52-3
- Min. Order:
- Purity: 99.93%
- Supply Ability: 10g
- Salicin
-

- $29.00 / 1mL
- 2024-10-31
- CAS:138-52-3
- Min. Order:
- Purity: 99.93%
- Supply Ability: 10g
- D-(-)-Salicin
-

- $0.00 / 1KG
- 2024-10-31
- CAS:138-52-3
- Min. Order: 1KG
- Purity: 98%min
- Supply Ability: 10tons/month