PCl5: Polarity, structure and properties
Dec 20,2023
Phosphorous pentachloride is an inorganic chemical compound with the molecular formula of PCl5. It mainly exists as a greenish-yellow crystalline solid. It has a strong irritating odour. The gaseous neutral molecule of phosphorous pentachloride has trigonal bipyramidal geometry due to its sp3d hybridization. It is one of the significant chlorides of phosphorous and is commonly used as a chlorinating agent and a catalyst in organic chemistry.

Polarity of PCl5
PCl5 is a non-polar compound. In its structure, the P-Cl bonds are polar but they are arranged in a symmetrical molecular geometry. As a result, the dipole moment of each bond gets cancelled out with no polar character left.
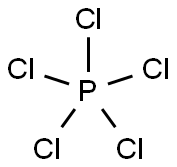
Structure of PCl5
It includes one phosphorus and five chlorine atoms. The chlorine atoms are connected to the phosphorus atom via five covalent (sigma) bonds.
Preparation of PCl5
The most common process used to prepare phosphorus pentachloride is the reaction between phosphorus trichloride and dry chlorine. The reaction is given as: PCl3(l)+Cl2(g)→PCl5(g).
Properties of PCl5
The physical and chemical properties of PCl5are discussed below:
Physical Properties
It exists as a greenish-yellow crystalline solid.
It has a molecular weight of 208.2 g/mol.
It has a density of 2.1g/cm3
It has a melting point of 160.5 °C (320.9 °F; 433.6 K).
It has a billing point of 166.8 °C (332.2 °F; 439.9 K).
It reacts with water and is soluble in CS2, chlorocarbons, and benzene.
Chemical Properties
It dissolves in water to form phosphoric acid and hydrochloric acid.
PCl5+4H2O→H3PO4+5HCl
Phosphoryl chloride and thionyl chloride are formed after a reaction between phosphorous pentachloride and sulphur dioxide.
PCl5+SO2→SOCl2+POCl3
- Related articles
- Related Qustion
Hexane is undoubtedly the most widely used among the solvents used industrially for extracting non-polar edible natural products such as colors, flavors, fragrances, or lipids.....
Dec 20,2023Organic SolventsPH3 is polar due to a lone pair of electrons with electron-electron repulsion, causing an overall "bent" structure. This results in a dipole moment throughout the molecule.....
Dec 20,2023Inorganic chemistryPhosphorus pentachloride
10026-13-8You may like
- Rhenium:Discovery,Minerals,Chemistry,Uses,Toxicity
May 31, 2024
- How are Lead Minerals Distributed?
May 31, 2024
- Discovery and Major Minerals of Bismuth
May 31, 2024
Phosphorus pentachloride manufacturers
- Phosphorus pentachloride
-

- $0.00 / 1kg
- 2023-10-09
- CAS:10026-13-8
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 20tons
- Phosphorus pentachloride
-

- $0.00 / 1KG
- 2023-09-06
- CAS:10026-13-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg
- phosphorus pentachloride
-

- $48.00 / 1KG
- 2023-05-04
- CAS:10026-13-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 200tons