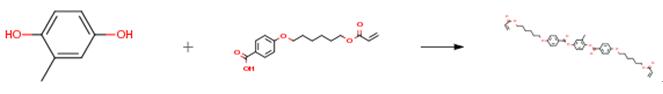
Preparation of 1,4-bis-[4-(6-acryloyloxyhexyloxy)benzoyloxy]-2-methylbenzene
Nov 27,2019
1,4-bis-[4-(6-acryloyloxyhexyloxy)benzoyloxy]-2-methylbenzene is an important organic intermediate to synthetize substituted benzene products.
1,4-bis-[4-(6-acryloyloxyhexyloxy)benzoyloxy]-2-methylbenzene can be prepared according to the reported literatures[1-2]
Method 1
4-(60-acryloxy-hexyloxy) benzoic acid (6.44 g, 0.022 mol) and DCC (5.96 g, 0.024 mol) were added to a round-bottom flask containing 60 mL CH2Cl2. Subsequently, the mixture of 2-methyl-hydroxy phenol (1.24 g, 10 mmol) and DMAP (0.28 g, 2 mmol) (dissolved in CH2Cl2) was dropped into the flask and stirred for two days at room temperature. After the reaction, the insoluble salts in the solution were removed by fltering. The obtained solution was further washed by HCl (5 wt% water solution) and NaHCO3 (5 wt% water solution) three times, in sequence. The residue was recrystallized with isopropanol over 5 times. Finally, the product as a white solid (C6M) was obtained.1H NMR (400 MHz, CDCl3-d, ppm): 8.11 (4H, t), 7.17–7.05 (3H, m), 6.96 (4H, q), 6.42 (1H, d), 6.38 (1H, d), 6.15–6.08 (2H, q), 5.84 (1H,d), 5.81 (1H, d), 4.17 (4H, t), 4.04 (4H, t), 2.23 (3H, s), 1.84 (4H, m), 1.72 (4H, m), 1.66–1.36 (8H, m).
Method 2

Ethylparaben (16.6 g, 0.1 mol), 6-chloro-1-hexanol (16.4 g, 0.12 mol), NaOH (4.0 g, 0.1 mol) and a small amount of KI were added to a stirred solution of butanone (300 ml). The mixture was stirred at 60 oC for 20 h, and then cooled to room temperature and filtered to remove the insoluble salts. Next, the mother liquor was evaporated and the residue was washed by 300 ml solution mixed with water and NaOH (0.4 mol/L). After that, the target product was extracted by ether from the solution and then dried in vacuum. At last, a white solid (4 - (6'-hydroxyhexyloxy) benzoate) was got. Yield 92%, 24 g. KOH (15 g, 0.375 mol) and 4 - (6'-hydroxyhexyloxy) benzoate (24 g, 0.9 mol) were added to 300 ml deionized water, and then stirred at 60 oC for 6 h. Next, the solution was extracted by ether and the water solution was left. After that, 20 ml HCl was added to the water solution and a white precipitation appear, and then the white precipitation was filtered and dried. At last, the precipitation was purified by recrystallization with ethanol twice and got a white solid (4 - (6'-hydroxy-hexyloxy) benzoic acid). Yield 80%, 17 g. N, N-dimethylaniline (7.27g, 0.06 mol) and 4 - (6'-hydroxy-hexyloxy) benzoic acid (14.3 g, 0.06 mol) were added to 300 ml 1,4-dioxane solution. Next, the mixture was heated to 60 oC, and then acryloyl chloride (5.4 g, 0.06 mol) was dropped and stirred for 3h. After that, the solution was poured into 1000 ml water and stirred. And then a precipitation was formed and was filtered. The precipitation was washed by water and purified by recrystallization with isopropanol twice, and then got a white solid 4 - (6'-acryloxy-hexyloxy) benzoic acid. Yield 78%, 13.7g. 4 - (6'-acryloxy-hexyloxy) benzoic acid (3.22 g, 0.011 mol) and N, N-dicyclohexyl carbodiimide (2.48 g, 0.012 mol) were added to 30 ml stirred DCM, and then 80 ml solution of DCM contained 2-methyl - hydroxy phenol (0.62 g, 0.005 mol) and 4-dimethylaminopyridine (0.14 g, 0.001 mol) was dropped into and stirred for 48 h. Next, the solution was filtered to remove the insoluble salts, then washed by HCl (5%) and aqueous sodium bicarbonate (5%) three times respectively, and then dried with magnesium. After that, the solution was filtered and the residue was purified by silica-gel column chromatography. At last, it was recrystallization with isopropanol twice, and then got white solid. Yield 70.5%, 2.4g.
References
1.Wang S, Zeng Q, Wang A, Liu X, Chen J, Wang Z, Zhang L. Constructing stable ordered ion channels for a solid electrolyte membrane with high ionic conductivity by combining the advantages of liquid crystal and ionic liquid[J]. Journal of Materials Chemistry A, 2019, 7(3):1069 – 1075.
2.Chen X, Wang L, Chen Y, Li C, Hou G, Liu X, Zhang X, He W, Yang H. Broadband reflection of polymer-stabilized chiral nematic liquid crystals induced by a chiral azobenzene compound[J]. Chemical Communications, 2014,50(6):691 – 694.
- Related articles
- Related Qustion
(S)-N-(Benzyloxycarbonyl)-N-methylvaline has been used as a reactant in the synthesis of a biologically active peptide. It is an important organic intermediate to synthetize substituted valine products.....
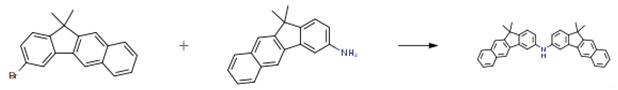
Nov 27,2019Amino Acids and Derivatives3-Bromo-11,11-dimethyl-11H-benzo[b]fluorene is an important organic intermediate to synthetize substituted benzo[b]fluorene products.....
Nov 27,2019Organic Synthesis IntermediateYou may like
1,4-BIS-[4-(6-ACRYLOYLOXYHEXYLOXY)BENZOYLOXY]-2-METHYLBENZENE manufacturers
- 1,4-Bis-[4-(6-acryloyloxyhexyloxy)benzoyloxy]-2-methylbenzene
-
![125248-71-7 1,4-Bis-[4-(6-acryloyloxyhexyloxy)benzoyloxy]-2-methylbenzene](/ProductImageEN/2023-08/Small/b7c59925-0016-421c-b7f6-f8d16c6fbc54.png)
- $0.00/ G
- 2024-01-02
- CAS:125248-71-7
- Min. Order: 10G
- Purity: 0.95
- Supply Ability: 50KG
- RM82 2-methyl-1,4-phenylene bis(4-((6-(acryloyloxy)hexyl)oxy)benzoate)
-

- $0.00 / 1kg
- 2022-09-20
- CAS:125248-71-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000kg
- 2-Methyl-1,4-phenylene bis(4-((6-(acryloyloxy)hexyl)oxy)benzoate)
-

- $1.00 / 1KG
- 2020-02-26
- CAS:125248-71-7
- Min. Order: 1KG
- Purity: 99%+
- Supply Ability: 100 tons