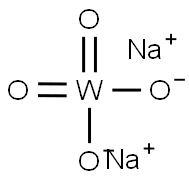Sodium Tungstate: An Overview of Its Mechanism and Applications in Chemistry
Apr 28,2024
Introduction
Sodium tungstate, a chemical compound with the formula Na2WO4, plays a crucial role in various industrial and chemical processes. This inorganic compound is well-known among chemists and industry professionals for its versatility and efficacy in numerous applications. As an odorless, white crystalline salt, sodium tungstate dissolves easily in water, making it a highly suitable candidate for aqueous solutions used in diverse chemical reactions. Its solubility and stability under various conditions enhance its utility in laboratories and industrial settings alike.

Figure 1 Characteristics of Sodium tungstate
Mechanism of action of Sodium tungstate
Sodium tungsten operates primarily through its ability to facilitate and catalyze various chemical reactions. As a source of tungsten ions, it acts as a mild oxidizing agent. In many chemical processes, the tungstate ion interacts with other substances to alter their chemical structure, typically enhancing their reactivity or modifying their oxidative state. This reactivity is crucial in the production of phosphors and organic synthesis where tungstate ions serve as catalysts. The mechanism by which sodium tungstate interacts with organic compounds often involves the transfer of electrons, which can lead to significant changes in molecular architecture and properties.
The efficiency of sodium tungstate as a catalyst is attributed to its stability and ability to form complex compounds with transitional states during reactions. These complexes often facilitate the transformation of reactants to products by lowering activation energies, thus increasing reaction rates. This property is especially valuable in industrial applications where speed and efficiency are paramount.
Application of Sodium tungstate
Sodium tungsten finds extensive use across a wide range of applications. In the medical field, it is used as a diagnostic agent for diabetes through its role in enhancing insulin activity and glucose metabolism. This application exploits the compound's ability to modulate enzymatic activities involved in glucose production and utilization, thereby providing a valuable tool for managing glycemic control.
In the realm of materials science, sodium tungsten is instrumental in the manufacture of fireproofing fabrics and anti-corrosive agents. The compound imparts fire resistance when applied to materials due to its chemical properties that inhibit combustion processes at a molecular level. Additionally, its application in metal treatments to prevent corrosion underlines its importance in extending the life and durability of metal products.
Another significant application of sodium tungstate is in the production of phosphors used in lighting and display technologies. The compound’s role in these processes involves facilitating the activation of luminescent materials that emit light under certain conditions, such as exposure to electrons or ultraviolet light[2].
Storage method for Sodium tungstate
The storage of sodium tungstate requires careful handling and specific conditions to maintain its chemical integrity and ensure safety. It should be stored in a cool, dry place away from direct sunlight and moisture, as these factors can lead to degradation of the compound and reduce its effectiveness. Sodium tungsten should also be kept in tightly sealed containers made of materials that do not react with tungsten ions, such as glass or certain plastics.
Proper labeling and storage in a designated chemical storage area are essential to avoid accidental ingestion or contact, given the compound’s potential toxicity. Handling procedures should always include the use of appropriate personal protective equipment, such as gloves and safety goggles, to prevent direct contact with the skin or eyes.
Conclusion
Sodium tungstate remains a pivotal substance in the field of chemistry due to its versatile applications and effective mechanism of action. From its use in healthcare and materials science to its role as a catalyst in organic synthesis, sodium tungstate demonstrates a remarkable capacity to enhance and enable a variety of chemical processes. Understanding its properties, applications, and proper storage methods is essential for professionals in the chemical industry, ensuring its effective and safe use in various sectors.
References
[1]Bertinat, Romina, et al. "Sodium tungstate: Is it a safe option for a chronic disease setting, such as diabetes?."Journal of Cellular Physiology234.1 (2019): 51-60.
[2]Roy, Sanchita, and Sanjay Bhar. "Sodium Tungstate-catalyzed “On-water” Synthesis of β-arylvinyl Bromides."Green Chemistry Letters and Reviews3.4 (2010): 341-347.
- Related articles
- Related Qustion
- Exploring the Potential of Sodium Tungstate with Antioxidant, Antidiabetic, and Adjuvant Therapy Jan 5, 2024
Sodium tungstate has potent antioxidant and antidiabetic effects, can enhance cancer treatment but requires further safety investigation.
- TUNGSTEN:Application and Toxicity Studies Feb 15, 2023
Tungsten is the bioelement with the highest atomic number 74, and the only bioelement in the third transition row of the periodic table.
Ethyl vanillin, a common flavoring, undergoes rapid metabolism, potentially causing toxicity at high doses, necessitating careful regulation for safety in food and drug applications.....
Apr 28,2024APIThe widespread application of Propylparaben as a preservative in the cosmetics, pharmaceutical, and food industries has attracted great attention.....
Apr 28,2024APISodium tungstate
13472-45-2You may like
Sodium tungstate manufacturers
- Sodium tungstate
-

- $220.00 / 10tons
- 2024-05-10
- CAS:13472-45-2
- Min. Order: 1tons
- Purity: 99%
- Supply Ability: 1000tons
- Sodium tungstate
-

- $6.00 / 1kg
- 2024-01-08
- CAS:13472-45-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000ton
- Sodium tungstate
-

- $30.00 / 1KG
- 2023-09-06
- CAS:13472-45-2
- Min. Order: 50KG
- Purity: 99%
- Supply Ability: 500000kg