Synthesis of 3,5-Dibromobenzaldehyde
Aug 23,2022
Physicochemical property
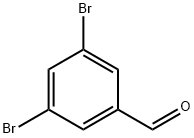
3,5-Dibromobenzaldehyde is a white or beige solid with a melting point of 84-88 °C. Its boiling point and density are estimated to be 287.2±20.0 °C and 1.977±0.06 g/cm3, respectively. It is insoluble in water.

Fig. 1 The structure of 3,5-Dibromobenzaldehyde.
Synthetic routes

Fig. 2 The synthetic method 1 of 3,5-Dibromobenzaldehyde.
Dissolve 2-((3,5-dibromobenzyl)sulfonyl)benzo[d]thiazole (0.1 mmol) and nitrosobenzene (0.2 mmol) in 1 mL of DMF. Stir the mixture until the solution presents a greenish color. Add Cs2CO3 (65 mg, 0.2 mmol, 2 equiv) to the solution. Stir the mixture at 50°C for 45 min. Cool the reaction mixture to room temperature. Extract the mixture with AcOEt (5 mL). Wash the mixture of the organic phase with water (2 x 5 mL) and brine (5 mL). Dry the reaction mixture over MgSO4. Remove the solvent under reduced pressure (hexane / AcOEt 3:1) to obtain the crude product. Add 1 mL of HCl 1M dropwise (bubbling was observed due to remaining carbonate salts) to the DMF solution of corresponding nitrone. Heat the mixture at 50°C. Stir the mixture for 1 h. Transfer the mixture to a separatory funnel. Extract the mixture with EtOAc (2 x 10 mL). Wash the organic layer with water (2 x 10 mL) and brine (10 mL). Dry the mixture over MgSO4. Remove the solvent under reduced pressure. Purify the crude with flash column chromatography (hexane/EtOAc = 30:1) to afford 3,5- dibromobenzaldehyde. 1H NMR (CDCl3, 300 MHz): δ 9.92 (s, 1H), 7.97-7.90 (m, 3H) [1].

Fig. 3 The synthetic method 2 of 3,5-Dibromobenzaldehyde.
The title compound was prepared following a modified literature method. 1,3,5-tribromobenzene (3.01 g, 9.6 mmol) in diethyl ether (80 mL) was cooled to -78°C followed by the addition of one equivalent of n-BuLi dropwise (2.5 M, 3.8 mL). The reaction was stirred for 30 minutes then DMF (740 μL, 9.6 mmol) was added dropwise to the reaction and stirred at -78°C for one hour. The vessel was then placed in an ice bath and stirred for 30 minutes. A 10% HCl solution (100 mL) was added to quench the reaction followed by CHCl3 (150 mL). The organic layer was collected and the aqueous layer washed with CHCl3 (80 mL). The organic layers where combined and dried over MgSO4 and the solvent removed. The crude product was purified by column chromatography eluting with 10% EtOAc in hexanes. Spectral data for the title compound was not reported in the literature reference. Yield: 1.93 g of the title compound (77%). 1H NMR (CDCl3, 300 MHz): δ = 9.90 (s, 1H), 7.92 (d, 2H), 7.60 (s, 1H); 13C NMR (CDCl3, 75 MHz) δ = 189.3, 139.7, 139.0, 131.37, 124.1; GC-MS [M+H]+ 262.8709, calcd 262.8707 [2].

Fig. 3 The synthetic method 3 of 3,5-Dibromobenzaldehyde.
Add 1 mL of HCl 1M dropwise (bubbling was observed due to remaining carbonate salts) to the DMF solution of corresponding nitrone. Heat the mixture at 50°C. Stir the mixture for 1 h. Transfer the mixture to a separatory funnel. Extract the mixture with EtOAc (2 x 10 mL). Wash the organic layer with water (2 x 10 mL) and brine (10 mL). Dry the mixture over MgSO4. Remove the solvent under reduced pressure. Purify the crude with flash column chromatography (hexane/EtOAc = 15:1) to afford 3,5-dibromobenzaldehyde. 1H NMR (CDCl3, 300 MHz): δ 9.92 (s, 1H), 7.97-7.90 (m, 3H) [3].
Precautions for the experiment
1. Before the experiment, wear protective glasses, protective clothing, mask, and gloves, and avoid contact with skin.
2. If toxic or irritating substances and harmful substances are encountered during the experiment, the experimental operation should be completed in the glove box when necessary to avoid causing harm to the experimenter.
3. The pipetting nozzle for taking samples should be replaced in time. If necessary, the filter cartridge suction head should be selected as far as possible to avoid cross contamination.
4. When weighing drugs, use weighing paper, take drugs and weigh them in a place without wind to avoid spreading. The container of reagents must be clean and disinfected before use.
5. When taking medicine, try to use multiple medicine spoons separately, clean them after use, dry them, disinfect them and store them.
6. Waste generated after the experiment shall be classified and stored and handed over to a professional biological waste gas treatment company to avoid environmental pollution.
References
[1] Rodrigo E, Alonso I, Cid M B. A protocol to transform sulfones into nitrones and aldehydes[J]. Organic letters, 2018, 20(18): 5789-5793.
[2] Sommer J R, Shelton A H, Parthasarathy A, et al. Photophysical properties of near-infrared phosphorescent π-extended platinum porphyrins[J]. Chemistry of Materials, 2011, 23(24): 5296-5304.
[3] Rodrigo E, Alonso I, Cid M B. A protocol to transform sulfones into nitrones and aldehydes[J]. Organic letters, 2018, 20(18): 5789-5793.
- Related articles
- Related Qustion
PF-07321332 (Nirmatrelvir) is a potent and orally active SARS-CoV?3C-like protease inhibitor.....
Aug 23,2022APIOxolinic acid is a quinolone antibiotic developed in Japan in the 1970s.It also acts as a dopamine reuptake inhibitor and has stimulant effects in mice.Dosages 12–20 mg/kg orally administered for five to ten days. The antibiotic works by in....
Aug 23,2022API3,5-Dibromobenzaldehyde
56990-02-4You may like
3,5-Dibromobenzaldehyde manufacturers
- 3,5-Dibromobenzaldehyde
-

- $100.00 / 1kg
- 2024-04-23
- CAS:56990-02-4
- Min. Order: 1kg
- Purity: 99.93%
- Supply Ability: 1000kg per week
- 3,5-Dibromobenzaldehyde
-
- $0.00 / 25KG
- 2024-03-28
- CAS:56990-02-4
- Min. Order: 25KG
- Purity: 95%
- Supply Ability: Inquiry
- 3,5-Dibromobenzaldehyde
-

- $35.00/ kg
- 2023-11-08
- CAS:56990-02-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20 Ton