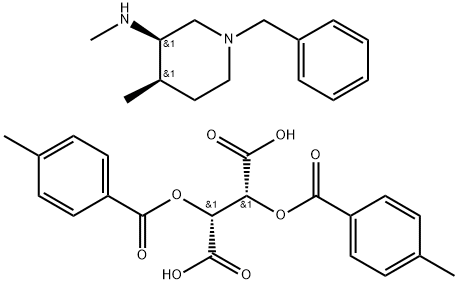
The introduction of 3-bis(4-Methylbenzoyloxy)succinate)
Jan 28,2023
General description
The 3-bis(4-Methylbenzoyloxy)succinate), with the CAS No: 477600-71-8, is also known as (2R,3R)-2,3-Bis[(4-methylbenzoyl)oxy]butanedioic acid compd. with (3R,4R)-N,4-dimethyl-1-(phenylmethyl)-3-piperidinamine. This chemical’s molecular formula is C34H40N2O8 and molecular weight is 604.7. The 3-bis(4-Methylbenzoyloxy)succinate) is a kind of synthetic intermediate. Its structure is as follows:

Figure 1 Structure of 3-bis(4-Methylbenzoyloxy)succinate).
Chemical synthesis of 3-bis(4-Methylbenzoyloxy)succinate)
3-bis(4-Methylbenzoyloxy)succinate) can be synthesized by 5 steps according to previous work [1]. Step 1: Toluene (1 L) and benzyl chloride (202 g, 1.60 mol) were added to the reaction mixture containing compound 15 and stirred for 15 min at room temperature. The temperature of the reaction mass was then increased to 75-85 °C and stirred for 8-10 has required to complete the reaction (monitored by TLC, mobile phase: chloroform/methanol = 8:2). The reaction mass was then cooled to room temperature. Water (1 L) was then added to the reaction mixture and separated out the aqueous layer. The aqueous layer was then cooled to 0-10 °C.
Step 2: Sodium borohydride solution (140 g, 3.70 mol, in 0.1 N sodium hydroxide) was then added to the aqueous layer dropwise at 0-10 °C and stirred for 10-12 has required to complete the reaction (monitored by TLC, mobile phase: chloroform/ methanol = 8:2). After the completion of the reaction, the precipitated solid was filtered by Buckner funnel. Compound 17: Yield = 360 g (82%); purity by reverse phase HPLC 95%. 1-Benzyl-4-methyl-1,2,5,6-tetrahydropyridin-3-ylacetylamine (17). MS m/z 245 (M+1)+; mp 112.8-114 °C; 1H NMR (CDCl3) δ 8.57 (s, 1H), δ 7.25-7.31 (m, 5H), δ 3.51 (s, 2H), δ 2.84 (s, 2H), δ 2.48 (t, 2H), δ 2.06 (t, 2H), δ 1.83 (s, 3H), δ 1.50 (s, 3H).
Step 3: Concentrated HCl 35% (600 mL, 2 volume) and 1-benzyl-4-methyl-1,2,5,6-tetrahydropyridin-3-ylacetylamine (17) (300 g, 1.23 mol) were charged in a 3 L four-neck round-bottom flask with an overhead stirrer and stirred for 10 min at room temperature. Temperature of the reaction mixture was then increased slowly to 65-70 °C and stirred for 3-4 h to complete the reaction (monitored by TLC, mobile phase: chloroform/methanol = 9:1). The reaction mixture was then cooled to 25-30 °C, and the pH of the mixture was adjusted to 8-12 by using 30% sodium hydroxide. The reaction mass was extracted with hexane (900 mL).
Step 4: The hexane layer was concentrated, and the resulting oily mass was dissolved in methanol (900 mL). The methanolic solution was cooled to 0-15 °C, and titanium(IV) tetraisopropoxide (385 mL, 1.30 mol) was added to the solution. Methanolic monomethylamine solution (33%, 200 mL, 2.12 mol) was added to the resulting complex, and the mixture was stirred at 5-15 °C for an hour. Sodium borohydride (46.5 g, 1.23 mol) was added to the reaction mixture in a small portion at 5-15 °C and stirred for an hour as monitored by TLC (mobile phase: chloroform/methanol = 8:2). The reaction mass was filtered off followed by distillation. Racemic crude amine.
Step 5: The crude amine was resolved by using L-ditoluoyl tartaric acid (240 g, 0.621 mol) in 1:1 mixture of methanol-water (2 L). The reaction mixture was stirred for an hour at 40-45 °C, and the precipitated product was filtered off. Compound 3: Yield = 168 g (37%); purity by chiral GC 98.6%. 3-bis(4-Methylbenzoyloxy)succinate) (3). MS m/z 219 (M+ 1)+; mp 202.2-203.6 °C; 1H NMR (MeOD) δ 8.04 (d, 2H, J = 8.0 Hz), δ 7.29 (m, 7H), δ 5.85 (s, 1H), δ 4.91 (s, 3H), δ 3.63 (d, 1H, J = 12.8 Hz), δ 3.42 (d, 1H, J = 12.8 Hz), δ 3.09 (s, 1H), δ 2.90 (m, 1H), δ 2.49 (s, 3H), δ 2.22 (m, 2H), δ 1.91 (m, 1H), δ 1.48-1.64 (m, 2H), δ 1.02 (d, 3H, J = 7.1 Hz).

Figure 2 Chemical synthesis of 3-bis(4-Methylbenzoyloxy)succinate).
Application of 3-bis(4-Methylbenzoyloxy)succinate)
3-bis(4-Methylbenzoyloxy)succinate) is a very important intermediate to synthesize chemical drug, especially tofacitinib. There are many routes for the synthesis of tofacitinib or its salt available in the literature [2]. The most important part for the preparation of tofacitinib is the synthesis of 3-bis(4-Methylbenzoyloxy)succinate), as it is very tedious and also requires very expensive reagent. It is the major cost-contributing factor for the synthesis of tofacitinib. There are several processes reported in the literature for the synthesis of 3-bis(4-Methylbenzoyloxy)succinate) [2-4].
References
[1]Patil et al. An Improved and Efficient Process for the Preparation of Tofacitinib Citrate. Organic Process Research & Development, 2014, 18(12): 1714-1720.
[2]Adolfo, M.; Mario, J. S.; Leonardo, S. S. Tetrahedron Lett. 2013, 54, 5096?5098.
[3]Stavber, G.; Cluzean, J. PCT WO 2014016338A1, 2014.
[4]Kristin, E. P.; Claude, L.-A.; Brett, M. L.; Robert, W. M.; Jason, M.; Kevin, W. H.; Jeol, M. H.; Rajappa, V. Org. Lett. 2009, 11, 2003?2006.
- Related articles
- Related Qustion
Selenium dioxide, SeO2 is an oxidizing agent generally employed in the allylic oxidation of alkenes to furnish allylic alcohols, which may be further oxidized to conjugated aldehydes or ketones.....
Jan 28,2023Inorganic chemistry3-bis(4-Methylbenzoyloxy)succinate)
477600-71-8You may like
3-bis(4-Methylbenzoyloxy)succinate) manufacturers
- 3-bis(4-Methylbenzoyloxy)succinate)
-
- $5.00 / 1KG
- 2024-04-16
- CAS:477600-71-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- 3-bis(4-Methylbenzoyloxy)succinate)
-
- $100.00 / 1KG
- 2019-07-06
- CAS:477600-71-8
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 100KG