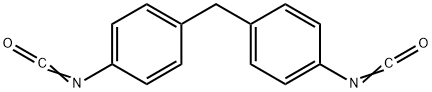
The production , Applications and Toxicokinetics of 4,4'-Diphenylmethane diisocyanate
Nov 30,2021
Production
Total world production of MDI and polymeric MDI is over 7.5 million tonnes per year (in 2017).
As of 2019, the largest producer was Wanhua Chemical Group.[3] Other major producers are Covestro,[4] BASF, Dow, Huntsman, Tosoh, Kumho Mitsui Chemicals. All major producers of MDI are members of the International Isocyanate Institute, whose aim is the promotion of the safe handling of MDI and TDI in the workplace, community and environment.
The first step of the production of MDI is the reaction of aniline and formaldehyde, using hydrochloric acid as a catalyst to produce a mixture of diamine precursors, as well as their corresponding polyamines:


Then, these diamines are treated with phosgene to form a mixture of isocyanates, the isomer ratio being determined by the isomeric composition of the diamine. Two different reaction mechanisms for this transformation are possible, namely "phosgenations first" and "step-wise phosgenations".[5]


Distillation of the mixture gives a mixture of oligomeric polyisocyanates, known as polymeric MDI, and a mixture of MDI isomers which has a low 2,4′ isomer content. Further purification entails fractionation of the MDI isomer mixture.[6]
Applications
The major application of 4,4′-MDI is the production of rigid polyurethane.[7] These rigid polyurethane foams are good thermal insulators and used in nearly all freezers and refrigeratorsworldwide, as well as buildings. Typical polyols used are polyethylene adipate (a polyester) and poly(tetramethylene ether) glycol (a polyether).
4,4′-MDI is also used as an industrial strength adhesive, which is available to end consumers as various high-strength bottled glue preparations.[8]
Safety
MDI is the least hazardous of the commonly available isocyanates, but is not benign.[9] Its very low vapour pressure reduces its hazards during handling compared to the other major isocyanates (TDI, HDI). However, it, like the other isocyanates, is an allergen and sensitizer. Persons developing sensitivity to isocyanates may have dangerous systemic reactions to extremely small exposures, including respiratory failure. Handling MDI requires strict engineering controls and personal protective equipment.[12] Compared to other organic cyanates, MDI has a relatively low human toxicity. It is a potentially violently reactive material towards water and other nucleophiles.
Reactivity of the isocyanate group
The positions of the isocyanate groups influences their reactivity. In 4,4′-MDI, the two isocyanate groups are equivalent but in 2,4′-MDI the two groups display highly differing reactivities. The group at the 4-position is approximately four times more reactive than the group at the 2-position due to steric hindrance.[4]


Toxicokinetics
There is very limited information on the toxicokinetics of MDI. Once absorbed, it is conjugated to protein. Only limited inhalation studies using rats are available. An inhalation exposure study using radiolabeled MDI indicates that MDI or derivatives are distributed throughout the body, predominantly in the lungs, muscle, kidneys, and digestive tract. The fecal and urinary elimination of MDI and its metabolites over 4 days was 57 and 13% of the recovered radioactivity, respectively. Less than 1% of the radioactivity was recovered from the major organs, although 23% of the administered dose was recovered in the carcass. In urine, small amounts of free and acetylated MDA were identified. Studies of workers have identified MDA with adducts in hemoglobin or albumin in urine and blood. The half-life of acid-hydrolyzable MDA in the urine of a worker exposed to PMDI was 70–80 h, and in serum, 21 days.
Mechanism of Toxicity
A definitive mechanism of MDI toxicity is unknown; however, it is speculated that humoral and cellular immunity may be involved in the pathogenesis of hypersensitivity due to isocyanate sensitization responses of both animals and humans.
References
1. Pubchem. "4,4'-Diphenylmethane diisocyanate | C15H10N2O2 - PubChem". pubchem.ncbi.nlm.nih.gov. Retrieved 2017-06-25.
2. Randall, D.; Lee, S. (2003). The Polyurethanes Book. New York: Wiley. ISBN 978-0-470-85041-1.
3. Tullo, Alexander H. (29 July 2019). "C&EN's Global Top 50 chemical companies of 2018". Chemical & Engineering News. Vol. 97 no. 30. Retrieved 15 January 2020.
4. Gal, J. (2012-02-20). "To the Rescue". ICIS Chemical Business. Archived from the original on 2012-03-26. Retrieved 2020-01-15.
5. "A theoretical study on the phosgenation of methylene diphenyl diamine (MDA)". Chemical Physics Letters. 706: 568–576. doi:10.1016/j.cplett.2018.06.024. ISSN 0009-2614.
6. Six, C.; Richter, F. "Isocyanates, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a14_611.
7. Boustead, I. (2005). "Polyurethane rigid foam" (PDF). Eco-Profiles of the European Plastics Industry. Brussels: PlasticsEurope. Archived from the original (PDF) on 2013-09-25.
8. US patent 6884904, Smith, A. K.; Goddard, R. J.; Paulsen, E. J. L., "MDI-based polyurethane prepolymer with low monomeric MDI content", issued 2005-04-26.
9. Allport, D. C.; Gilbert, D. S.; Outterside, S. M., eds. (2003). MDI and TDI: Safety, Health and the Environment: A Source Book and Practical Guide. Wiley. ISBN 978-0-471-95812-3.
- Related articles
- Related Qustion
- 4,4'-Diphenylmethane diisocyanate: uses and Hazard Oct 12, 2024
4,4'-Diphenylmethane diisocyanate is a light yellow coloured solid. It is not soluble in water. It may be toxic by ingestion, inhalation, or skin absorption.
Butadiene is produced during the combustion of organic matter. Significant amounts of butadiene are released to the environment from both natural and anthropogenic sources such as forest fires, gasoline and diesel engine exhaust, and wood s....
Nov 30,2021Organic ChemistryVitamin E exists in eight different forms, four tocopherols and four tocotrienols. All feature a chromane ring, with a hydroxyl group that can donate a hydrogen atom to reduce free radicals and a hydrophobic side chain.....
Nov 30,2021Vitamins and Minerals medicines4,4'-Diphenylmethane diisocyanate
101-68-8You may like
- Is benzoic acid polar or nonpolar?
Dec 21, 2023
- Hazards to the Environment and Humans of Ethyl Acetate
Nov 16, 2022
- Ethyl acetate - Uses, Properties, Safety etc.
Dec 29, 2021
4,4'-Diphenylmethane diisocyanate manufacturers
- Methylene diphenyl diisocyanate
-

- $0.00 / 200kg
- 2024-10-29
- CAS:101-68-8
- Min. Order: 200kg
- Purity: 94%
- Supply Ability: 20 tons
- 4,4'-Diphenylmethane diisocyanate
-

- $50.00 / 1kg
- 2024-10-24
- CAS:101-68-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20Tons
- 4,4'-Diphenylmethane diisocyanate
-
- $50.00 / 1KG
- 2023-12-23
- CAS:101-68-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available