What is Perchloroethylene?
Sep 27,2021
General description
Perchloroethylene (C2Cl4, CAS No: 127-18-4), also known as perc, is a colorless, nonflammable liquid solvent with a sweet, ether-like odor. It is primarily used in industrial settings and also for dry-cleaning fabrics and degreasing metals. Perchloroethylene is a non-flammable, multipurpose solvent that is relatively inert and inherently more stable than other chlorinated solvents (1).


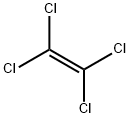
Figure 1. Molecular formula of perchloroethylene
Tetrachloroethylene dissolves in water forms dichloromethane and oxygen. The chemical equation is given below (1,2):
C2Cl4 + 2H2O → 2CH2Cl2 + O2
Tetrachloroethylene reacts with base like sodium hydroxide resulting in the formation of formic acid and sodium chloride (1,2):
4NaOH + C2Cl4 → 2HCO2H + 4NaCl
Application
Perchloroethylene offers many physical and chemical properties that make it the right chlorinated solvent for many applications (1). It is relatively inert and inherently more stable than other chlorinated solvents. Perchloroethylene’s high solvency and high vapor density make it ideal for a variety of end uses, and as a result, perchloroethylene has become the largest volume dry cleaning solvent and the choice for vapor degreasing (3, 4).
The major industrial uses of tetrachloroethylene are as a solvent in dry-cleaning, as a degreasing agent for manufactured metal parts and as a precursor in the production of chlorofluorocarbons. Other applications include the finishing and processing of textiles, the production of paint removers and printing ink, and the formulation of adhesives and specialized cleaning fluids. Consumer products that contain tetrachloroethylene include motor vehicle cleaners, stain removers, adhesives and wood cleaners (3,4). Details are summarized as below (3,4):
Dry cleaning - Perchloroethylene is the preferred solvent because, in addition to its non-flammability, it provides a fast, powerful, yet gentle cleaning action with a minimum of mechanical agitation.
Vapor Degreasing - Many industries, including aerospace, automotive, and household appliance production, use perchloroethylene in vapor degreasing for metal parts. Perchloroethylene is ideal for situations that require a high boiling point (above that of water). Many soils, such as waxes and resins, must be melted in order to be solubilized, making perchloroethylene a preferred solvent.
Chemical Processing - Perchloroethylene serves as a carrier solvent for fabric finishes, rubber, and silicones. It also is used as an extractant solvent in paint removers and printing inks. It serves as a chemical intermediate in many applications. As with all applications, when using perchloroethylene to decrease the flammability of a mixture, it is important to determine the flash point of the final product as it is to be used prior to selling, since an insufficient quantity of perchloroethylene will not raise the flash point of the mixture.
Catalyst Regeneration - Perchloroethylene is used in the petroleum refinery industry as a source of hydrochloric acid, a promoter, which helps in the regeneration of catalyst in both catalytic reformer and isomerization operations. Product sold into this operation must be a purer, less stabilized grade than most to preclude the poisoning of the platinum catalyst.
Fluorocarbon - Perchloroethylene is used in the manufacture of refrigerants, refrigerant blends, and other fluorinated compounds.
Sources and Preparation
There are no known natural sources of tetrachloroethylene. The estimated production volume in the United States of America in 1990 was 137 kilotonnes, compared to 282 kilotonnes in 1980 (5). The corresponding figures for western Europe and Japan for 1990 were 280 and 83 kilotonnes, respectively (3). The estimated annual consumption in western Europe for 1990 was 235 kilotonnes; for the United States and Japan it was 178 and 102 kilotonnes, respectively (5).
Perchloroethylene could be prepared through the high temperature oxyhydrochlorination of chlorinated hydrocarbons (6). Vaporized chlorinated organics are mixed with hydrochloric acid and oxygen inside a catalytic fluidized bed reactor where a series of chlorination and thermal cracking reactions occur. The overall exothermic reaction is carefully controlled to maintain the required temperature. The resulting product is a blend of trichloroethylene and perchloroethylene, which is distillation separated. Perchloroethylene is then further purified by distillation and stabilized to prevent oxidation.
Toxicity and safety
Effects resulting from acute (short term) high-level inhalation exposure of humans to tetrachloroethylene include irritation of the upper respiratory tract and eyes, kidney dysfunction, and neurological effects such as reversible mood and behavioral changes, impairment of coordination, dizziness, headache, sleepiness, and unconsciousness. The primary effects from chronic (long term) inhalation exposure are neurological, including impaired cognitive and motor neurobehavioral performance. Tetrachloroethylene exposure may also cause adverse effects in the kidney, liver, immune system and hematologic system, and on development and reproduction(2). Studies of people exposed in the workplace have found associations with several types of cancer including bladder cancer, non-Hodgkin lymphoma, multiple myeloma. EPA has classified tetrachloroethylene as likely to be carcinogenic to humans.
References
1. Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Tetrachloroethylene (Update). U.S. Public Health Service, U.S. Department of Health and Human Services, Atlanta, GA. 1997.
2. U.S. Environmental Protection Agency. Integrated Risk Information System (IRIS) on Tetrachloroethylene. National Center for Environmental Assessment, Office of Research and Development, Washington, DC. 2012.
3. Dry cleaning, some chlorinated solvents and other industrial chemicals. Lyon, International Agency for Research on Cancer, 1995 (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 63).
4. Tetrachloroethylene. Priority substances assessment report for the Canadian Environmental Protection Act. Ottawa, Environment Canada and Health Canada, 1993.
5. Toxicological profile for tetrachloroethylene. Atlanta, GA, Agency for Toxic Substances and Disease Registry, 1993 (Public Health Service Report, No. TP-92/18).
6. Preliminary Information on Manufacturing, Processing, Distribution, Use, and Disposal, February 2017 Office of Chemical Safety and Pollution Prevention U.S. EPA.
- Related articles
- Related Qustion
- Tetrachloroethylene: Uses and Effects on humans Mar 20, 2024
Tetrachloroethylene is mainly used as a dry-cleaning agent. It is an intermediate for production of trichloroacetic acid and fluorine-containing organic compounds.
- Biological actions and interactions of tetrachloroethylene May 7, 2022
Tetrachloroethylene is a colorless, volatile, nonflammable, liquid, chlorinated hydrocarbon with an ether-like odor that may emit toxic fumes of phosgene when exposed to sunlight or flames with a char
Silodosin is a selective antagonist of alpha(α)-1 adrenergic receptors that binds to the α1A subtype with the highest affinity. α1-adrenergic receptors regulate smooth muscle tone in the bladder neck, prostate, and prostatic urethra: the α1....
Sep 24,2021APICiticoline Sodium is generally used to treat various central nervous system diseases caused by accidents of brain injury and the appearance of cardiovascular and cerebrovascular diseases.....
Sep 27,2021APITetrachloroethylene
127-18-4You may like
Tetrachloroethylene manufacturers
- Tetra Chloroethylene
-

- $10.00 / 25kg
- 2024-10-31
- CAS:127-18-4
- Min. Order: 1kg
- Purity: 99.5%
- Supply Ability: 100
- Perchloroethylene
-

- $0.00 / 200kg
- 2024-10-29
- CAS:127-18-4
- Min. Order: 20kg
- Purity: 99.0%
- Supply Ability: 20 tons
- Tetrachloroethylene
-

- $1.00 / 300KG
- 2024-10-25
- CAS:127-18-4
- Min. Order: 300KG
- Purity: 99(%)
- Supply Ability: 2000 tons