Zeolite: The Versatile Material Shaping Modern Chemistry
May 15,2024
Introduction
Zeolites, a group of unique and naturally occurring minerals, are aluminosilicate compounds characterized by their stable and microporous structures. These minerals are not only fascinating from a geological perspective but have also become pivotal in various industrial and scientific applications due to their unique chemical properties[1].

Figure 1 Characteristics of Zeolite
Properties of Zeolite
Zeolites are renowned for their remarkable properties, which make them indispensable in several chemical processes. The defining characteristic of zeolites is their well-defined and uniform pore structure, which can be adjusted during synthesis to suit specific needs. These pores allow zeolites to act as molecular sieves, selectively adsorbing molecules based on size and shape.
Thermally stable and resistant to degradation in a variety of chemical environments, zeolites can withstand high temperatures without collapsing their framework. This thermal stability, combined with their high surface area and adsorption capacity, makes them effective catalysts and adsorbents.
Another notable property is their ability to exchange cations within their structure. Zeolites can swap the cations present in their framework with those in a surrounding solution without altering their structure. This ion exchange capacity is essential for applications in water softening, heavy metal removal, and other environmental remediation processes.
Main Components of Zeolite
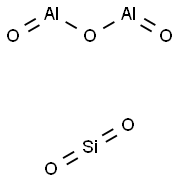
The primary components of zeolites are aluminum, silicon, and oxygen, which form a three-dimensional framework of SiO4 and AlO4 tetrahedra. The negative charge of the framework, due to the presence of aluminum, is balanced by the inclusion of cations such as sodium, potassium, calcium, and magnesium.
In natural zeolites, these cations are often found in varying proportions, contributing to the diversity of zeolite structures. There are over 50 different naturally occurring zeolite frameworks, and hundreds more have been synthesized in the lab, each with unique structural and chemical characteristics. The most common natural zeolites include clinoptilolite, mordenite, and natrolite, while synthetic varieties often have tailored structures for specific applications.
Applications of Zeolite
The applications of zeolites are vast and varied, cutting across multiple industries from medicine to manufacturing[2].
Catalysis
One of the most significant uses of zeolites is as catalysts in petrochemical refineries. They are crucial for processes like fluid catalytic cracking (FCC) and hydrocracking, where they help break down large hydrocarbon molecules into gasoline, diesel, and other petroleum products. Zeolites' ability to selectively adsorb molecules allows for more efficient and specific product outcomes in these reactions.
Adsorption and Ion Exchange
Zeolites are also used in adsorption processes where their porous structure and high surface area make them excellent for trapping contaminants. In water treatment, zeolites are employed to remove heavy metals, ammonium ions, and other pollutants, improving water quality and safety.
Their ion-exchange properties are particularly useful in detergents, where they replace sodium ions with calcium and magnesium to soften water. This process enhances the effectiveness of the detergent and prevents the buildup of minerals on fabrics and washing machines.
Agriculture and Aquaculture
In agriculture, zeolites are used to improve soil quality and water retention. They can release nutrients slowly, which enhances plant growth without the risk of over-fertilization. In aquaculture, zeolites help maintain water quality by adsorbing ammonia and other toxic compounds, ensuring a healthier environment for aquatic life.
Medical and Health Applications
In the medical field, zeolites are finding their place in wound care products due to their ability to adsorb fluids and odors. Research is also ongoing into their potential use in drug delivery systems, where their porous structure could be used to encapsulate drugs, allowing for controlled release over time.
Energy Storage and Conversion
Zeolites contribute to energy efficiency and storage, particularly in adsorption heat pumps and batteries. Their ability to adsorb and desorb water vapor can be used to drive thermal processes, making them valuable in renewable energy systems. In addition, their ion exchange and structural properties are being explored for use in batteries and fuel cells.
Transportation of Zeolite
The transportation of zeolites is straightforward due to their stability and non-hazardous nature. Typically, natural zeolites are mined and transported in bulk using standard shipping methods, including trucks, trains, and ships. For synthetic zeolites, which are often produced in pellet or powder form, transportation involves secure packaging to prevent moisture absorption and contamination.
Handling large quantities of zeolites requires considerations for dust control and packaging integrity. Industrial-grade zeolites are usually shipped in lined or coated containers to protect them from moisture, which can affect their adsorptive properties.
References
[1]Ozin G A, Kuperman A, Stein A. Advanced zeolite, materials science[J]. Angewandte Chemie International Edition in English, 1989, 28(3): 359-376.
[2]Van Speybroeck V, Hemelsoet K, Joos L, et al. Advances in theory and their application within the field of zeolite chemistry[J]. Chemical Society Reviews, 2015, 44(20): 7044-7111.
- Related articles
- Related Qustion
- Titanium Silicalite-1 - TS-1 Catalyst Mar 16, 2023
Titanium silicalite-1 is widely used in industry owing to its ability to catalytically epoxidize olefins with hydrogen peroxide (H2O2).
- Characteristic and Uses of Titanium Silicalite-1 Jun 13, 2022
Titanium Silicalite-1, TS-1, was the first framework-substituted redox molecular sieve to be identified. Redox molecular sieves offer a significant advantage over standard heterogenous catalysts; a redox sieve makes it possible to get a sig
- What is Zeolite? Mar 14, 2022
Zeolite, any member of a family of hydrated aluminosilicate minerals that contain alkali and alkaline-earth metals. The zeolites are noted for their lability toward ion-exchange and reversible dehydration.
Trifluoromethyl iodide, often abbreviated as CF3I, is a remarkable chemical compound that has garnered significant attention within the scientific community.....
May 15,2024APIHydroxyapatite is a naturally occurring mineral form of calcium apatite and is a versatile and invaluable material in the fields of chemistry and biomedicine.....
May 15,2024APIZeolite
1318-02-1You may like
- Zeolite
-

- $80.00 / 1kg
- 2024-05-30
- CAS:1318-02-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20Tons
- Zeolite
-

- $6.00 / 1kg
- 2024-05-16
- CAS:1318-02-1
- Min. Order: 1kg
- Purity: More than 99%
- Supply Ability: 2000KG/Month
- Zeolites other than erionite (clinoptilolite, phillipsite,mordenite, non-fibrous Japanese zeolite, synthetic zeolites)
-

- $100.00 / 1KG
- 2024-05-15
- CAS:1318-02-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20