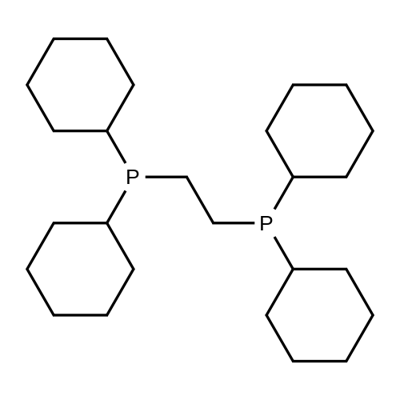
1,2-BIS(DICYCLOHEXYLPHOSPHINO)ETHANE synthesis
- Product Name:1,2-BIS(DICYCLOHEXYLPHOSPHINO)ETHANE
- CAS Number:23743-26-2
- Molecular formula:C26H48P2
- Molecular Weight:422.62

106-93-4
376 suppliers
$15.00/5g
829-84-5
140 suppliers
$24.19/1 g

23743-26-2
130 suppliers
$80.00/250 mg
Yield:23743-26-2 81%
Reaction Conditions:
Stage #1:dicyclohexylphosphane with cesiumhydroxide monohydrate in N,N-dimethyl-formamide at 20; for 1 h;Molecular sieve;Inert atmosphere;
Stage #2:ethylene dibromide in N,N-dimethyl-formamide at 20; for 49 h;Inert atmosphere;Molecular sieve;
Steps:
9
Comparative Catalyst Preparation Example 9Preparation of (cyclohexyl)2PCH2CH2P(cyclohexyl)2 ligand; A (cyclohexyl)2PCH2CH2P(cyclohexyl)2 ligand was prepared by reacting diphenylphosphine with 2 equivalents of dibromoalkyl in dimethylfluoromethylene (DMF) and cesium hydroxide atmospheres, as disclosed in the document “R. N. Salvatore et al, Tetrahedron Letters 44 (2003) 8373”. First, 360 mg (2.14 mmol) of a cesium hydroxide monohydrate was added to 16.6 ml of an anhydrous N,N-dimethylformamide suspension mixed with 1.0 g of activated molecular sieve powder having a particle size of 4 , and was then stirred in a nitrogen atmosphere. Subsequently, 0.43 ml (2.14 mmol) of dicyclohexyl phosphine was added thereto, and was then stirred at room temperature for 1 hour to form a dark reddish orange solution. 0.11 ml (1.29 mmol) of 1,2-dibromoethane was dropped into the solution, which thus became white. The solution was reacted for 49 hours at room temperature, and 60 ml of distilled water was added thereto, and the solution was extracted three times using 60 ml of DMC to form an organic layer. The organic layer was washed three times with distilled water, and was dried using anhydrous sodium sulfate, the solvent was removed therefrom in a vacuum, and then the organic layer, from which the solvent had been removed, was recrystallized in a benzene solvent, thereby obtaining air-sensitive white crystals (366 mg, yield 81%).
References:
Han, Taek Kyu;Ok, Myung Ahn;Chae, Sung Seok;Kang, Sang Ook;Jung, Jae Ho US2010/137669, 2010, A1 Location in patent:Page/Page column 12
16527-12-1
0 suppliers
inquiry

23743-26-2
130 suppliers
$80.00/250 mg

16523-54-9
250 suppliers
$8.00/1g

23743-26-2
130 suppliers
$80.00/250 mg