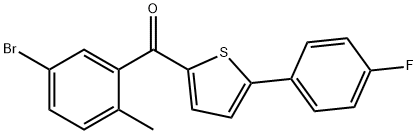
(5-broMo-2-Methylphenyl)(5-(4-fluorophenyl)thiophen-2-yl)Methanone synthesis
- Product Name:(5-broMo-2-Methylphenyl)(5-(4-fluorophenyl)thiophen-2-yl)Methanone
- CAS Number:1132832-75-7
- Molecular formula:C18H12BrFOS
- Molecular Weight:375.25
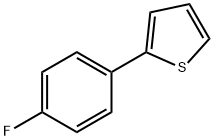
58861-48-6
352 suppliers
$8.00/1g
79669-49-1
436 suppliers
$5.00/1g

1132832-75-7
142 suppliers
inquiry
Yield:1132832-75-7 93.2%
Reaction Conditions:
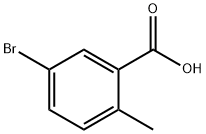
Stage #1:5-bromo-2-methylbenzoic acid with thionyl chloride in dichloromethane;N,N-dimethyl-formamide at 20 - 25;
Stage #2:2-(4-fluorophenyl)thiophene with aluminum (III) chloride in dichloromethane at 0 - 25; for 12.5 h;Solvent;Reagent/catalyst;
Steps:
3.2 (2) Preparation of 2-(5-bromo-2-methylbenzoyl)-5-(4-fluorophenyl) thiophene
into the reaction flask add 30g of 5-bromo-2-methylbenzoic acid, 6mL DMF and 400mL Dichloromethane, stirring. Under the room temperature added the drops of 24.6g of thionyl chloride; after the drop is completed, the temperature is increased at 20-25 °C; the reaction is monitored by TLC. After the reaction is completed, 25.2g of anhydrous aluminum chloride are added portion-wise into the reaction flask, stir for 30min, and cool down at 0-5 ° C, add the drops of 26.5g of a dichloromethane solution of 2-(4-fluorophenyl)-thiophene; after dripping, warm up to 25-30 °C and carry on reaction for 12 hours, TLC tracking reaction. After the reaction is over, the reaction solution is poured into 800mL ice water and quenched, stirring, static stratification. The aqueous phase is extracted with 300mL of dichloromethane; combine the organic phase, concentrated and then obtained Brown solid. The above solid is heated and dissolved with 8OOmL ethyl acetate, added the drops of 200mL of n-hexane, the crystals are stirred, filtered, and dried and then obtained 48.7g of a yellow solid product, yield is 93.2%.
References:
Nantong Changyou Pharmaceutical Technology Co., Ltd.;Li Zebiao;Hu Haiyang;Ding Haiming;Gu Wenchao;Mao Qing;Yan Jun CN104926803, 2017, B Location in patent:Paragraph 0037; 0048; 0058-0059

58861-48-6
352 suppliers
$8.00/1g

21900-41-4
25 suppliers
$120.68/Price $/250mgs:

1132832-75-7
142 suppliers
inquiry

1073313-97-9
15 suppliers
inquiry

842135-03-9
6 suppliers
inquiry

1132832-75-7
142 suppliers
inquiry
460-00-4
607 suppliers
$6.00/10g

1132832-75-7
142 suppliers
inquiry

79669-49-1
436 suppliers
$5.00/1g

1132832-75-7
142 suppliers
inquiry