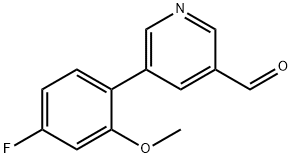
5-(4-Fluoro-2-methoxyphenyl)pyridine-3-carboxaldehyde synthesis
- Product Name:5-(4-Fluoro-2-methoxyphenyl)pyridine-3-carboxaldehyde
- CAS Number:1329115-52-7
- Molecular formula:C13H10FNO2
- Molecular Weight:231.22
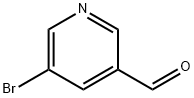
113118-81-3
277 suppliers
$5.00/250mg
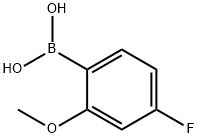
179899-07-1
197 suppliers
$9.00/250mg

1329115-52-7
10 suppliers
inquiry
Yield:1329115-52-7 62%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;caesium carbonate in 1,2-dimethoxyethane;water;toluene at 80; for 12 h;Suzuki coupling;
Steps:
Synthesis of 6a
5-bromonicotinaldehyde (1.80 g, 9.67 mmol) was dissolved in DME/EtOH/H2O (7:2:1, 1 M, 10 mL) and treated with 4-fluoro-2-methoxyphenylboronic acid (1.64 g, 9.67 mmol) followed by cesium carbonate (3.43 g, 10.54 mmol) and PdCl2(dppf)-CH2Cl2 (0.32 g, 0.39 mmol). The reaction mixture was heated at 80 °C for 12 h and then partitioned between EtOAc and H2O. The organic layer was washed with saturated NaHCO3, saturated NaCl, dried over MgSO4, filtered and concentrated under reduced pressure to give a dark brown solid. Chromatography (SiO2, 0 - 50% ethyl acetate in hexanes) yielded the desired product as a white solid (1.38 g, 62% yield). 1H NMR (300 MHz, CDCl3-d) δ ppm 3.83 (s, 3 H) 6.66 - 6.91 (m, 2 H) 7.19 - 7.40 (m, 1 H) 8.26 (t, J = 2.11 Hz, 1 H) 9.00 (d, J = 2.11 Hz, 1 H) 8.94 (d, J = 2.32 Hz, 1 H) 10.17 (s, 1 H); m/z (ES+)M+1 = 231. The resultant aldehyde (0.300 g, 1.29 mmol) was dissolved in dichloromethane (3 mL) along with cyclopentyl amine (0.166 g, 0.192 mL, 1.94 mmol). The solution was cooled in an ice bath, and to this was added sodium triacetoxy borohydride (0.310 g, 1.50 mmol). The reaction was stirred at room temperature for 16 h and diluted with dichloromethane (50 mL). The resultant mixture was then washed with saturated NaHCO3 (1 x 20 mL) and brine (1 x 20 mL). The combined organics were concentrated, and the residual oil purified by SiO2 chromatography (0 - 5% MeOH/DCM) to give 6a as a yellow oil. (0.12 g, 31%).
References:
Brown, Dean G.;Maier, Donna L.;Sylvester, Mark A.;Hoerter, Tiffany N.;Menhaji-Klotz, Elnaz;Lasota, Celina C.;Hirata, Lee T.;Wilkins, Deidre E.;Scott, Clay W.;Trivedi, Shephali;Chen, Tongming;McCarthy, Dennis J.;MacIag, Carla M.;Sutton, Evelynjeane J.;Cumberledge, Jerry;Mathisen, Don;Roberts, John;Gupta, Anshul;Liu, Frank;Elmore, Charles S.;Alhambra, Cristobal;Krumrine, Jennifer R.;Wang, Xia;Ciaccio, Paul J.;Wood, Michael W.;Campbell, James B.;Johansson, Magnus J.;Xia, Jian;Wen, Xiaotian;Jiang, Ji;Wang, Xiaoping;Peng, Zuozhong;Hu, Tao;Wang, Jian [Bioorganic and Medicinal Chemistry Letters,2011,vol. 21,# 11,p. 3399 - 3403] Location in patent:scheme or table