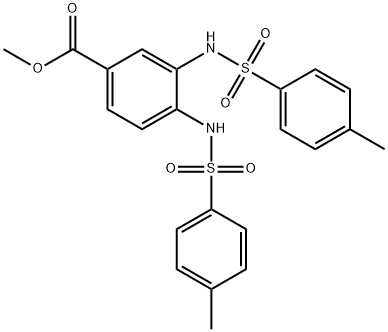
METHYL 3,4-DI[[(4-METHYLPHENYL)SULFONYL]AMINO]BENZOATE synthesis
- Product Name:METHYL 3,4-DI[[(4-METHYLPHENYL)SULFONYL]AMINO]BENZOATE
- CAS Number:175204-19-0
- Molecular formula:C22H22N2O6S2
- Molecular Weight:474.55
Yield:175204-19-0 62.6%
Reaction Conditions:
with pyridine;4-pyrrolidin-1-ylpyridine in acetonitrile; for 72 h;Inert atmosphere;Reflux;
Steps:
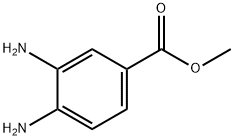
2 Example 2: Synthesis of methyl 3,4-diamino-/V, /V-b/'s-(toluene-4- sulfonylamino)benzoate (12)
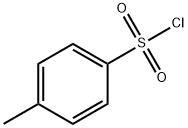
Methyl 3, 4-diaminobenzoate (10) (1.0 g, 0.006 mol), p-toluenesulfonyl chloride (11 ) (2.52 g, 0.013 mol) and 4-pyrrolidinopyridine (0.90 g, 0.006 mol) were added to dry acetonitrile (25 mL) under argon. Dry pyridine (2.4 mL, 0.03 mol) was then added and the resulting solution was heated at reflux for 72 hours. After this time, the acetonitrile was removed invacuo to afford a brown oily residue. The residue was dissolved in dichloromethane (DCM) (30 mL) and washed with 0.5 M HCI (3 χ 30 mL). The organic layer was dried (MgS04) and the solvent removed invacuo to yield a brown powder, which was purified by column chromatography on silica, eluting with DCM : methanol (95 : 5) to afford crudemethyl 3,4-diamino-6/'s-(toluene-4-sulfonylamino)benzoate(12). This was recrystallised from DCM : petroleum ether to yield pure methyl 3,4-diamino-/5/'s- (toluene-4-sulfonylamino)benzoate (12) as brown crystals (1.78 g, 62.6 %); mp 175 °C; Rf = 0.44 (DCM : methanol, 9 : 1); m/z 496.9 (MNa+); Analysis Calcd. for C22H22N2O6S2: C, 55.68; H, 4.67; N, 5.90 %. Found C, 55.86; H, 4.70; N, 5.74 %; vmax (KBr) / cm"1 3217 (NH), 1704 (C=0), 1157 (S=0); δΗ (d6-DMSO, 300 MHz) 9.62 (2H, s, 2 NH), 7.67 (2H, d, J= 8.4,H2J H6. orH2- /H6-), 7.62 (1 H, dd, J6, 5 = 8.4, J6, 2 = 1.8, H6), 7.57 (3H, m, H2 and Η2· / Η6. ΟΓ H2- / H6-),7.36 (4H, d, J= 8.4, H3'/ H5. andH3"/ H5 FontWeight="Bold" FontSize="10" ) 7.26 (1 H, d, J5, e = 8.4, H5), 3.74 (3H, s, OCH3), 2.36 (3H, s, CH3), 2.35 (3H, s, CH3);5C (d6-DMSO, 75 MHz)165.5 (CO), 144.5 (C4 and C4"), 144.3 (C4), 136.4 (Cr and C ), 135.8 (C3), 130.3 (C3J C5. or C3 FontWeight="Bold" FontSize="10" / C5 ), 130.3 (C3J C5' or C3"/ C5"), 127.7 (C6), 127.4 (C2J C6. or C2 FontWeight="Bold" FontSize="10" / Ce FontWeight="Bold" FontSize="10" ), 127.4 (C2J Ce- or C2- / C6"), 125.7 (C2), 125.6 (d), 120.5 (C5), 52.6 (OCH3), 21.5 (2 CH3).
References:
WO2014/96864,2014,A1 Location in patent:Paragraph 00177
619-05-6
289 suppliers
$19.00/25g
![METHYL 3,4-DI[[(4-METHYLPHENYL)SULFONYL]AMINO]BENZOATE](/CAS/GIF/175204-19-0.gif)
175204-19-0
25 suppliers
inquiry