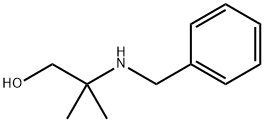
2-Benzylamino-2-methyl-1-propanol synthesis
- Product Name:2-Benzylamino-2-methyl-1-propanol
- CAS Number:10250-27-8
- Molecular formula:C11H17NO
- Molecular Weight:179.26
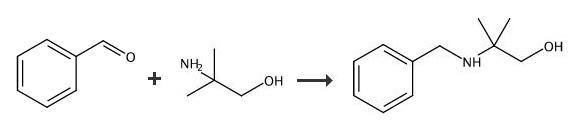
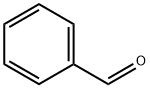
100-52-7
948 suppliers
$17.30/2g
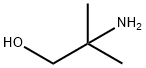
124-68-5
520 suppliers
$4.76/25g

10250-27-8
110 suppliers
$89.00/1g
Yield:10250-27-8 96%
Reaction Conditions:
Stage #1:benzaldehyde;2-Amino-2-methyl-1-propanol in dichloromethane at 20; for 3 h;Molecular sieve;
Stage #2: with methanol;sodium tetrahydroborate at 20; for 1 h;
Steps:
9.a (a) 2-(Benzylamino)-2-methylpropan-1-ol
Benzaldehyde (3.4 mL, 33.7 mmol) was added dropwise to a stirred mixture of 2-amino- 2-methylpropan-1-ol (3.0 g, 33.7 mmol), 5 A molecular sieves (5 g) and CH2CI2(30 mL) at rt. The mixture was stirred at rt for 3 h, filtered through a pad of cotton and concentrated. MeOH (20 mL) followed by NaBH4(1.5 g, 40.4 mmol) was added and the mixture was stirred at rt for 1 h. NH4CI (aq, sat, 10 mL) was added and the mixture was concentrated, treated with NaOH (1 M, 20 mL) and extracted with EtOAc. The combined extracts were dried (Na2SO4) and concentrated to give the sub-title compound (5.8 g, 33.3 mmol, 96 %), which was used in the next step without further purification.
References:
ATROGI AB;PELCMAN, Benjamin;BENGTSSON, Tore WO2020/188299, 2020, A1 Location in patent:Page/Page column 59; 60
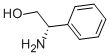
20989-17-7
451 suppliers
$5.00/1g

100-52-7
948 suppliers
$17.30/2g

10250-27-8
110 suppliers
$89.00/1g

20515-61-1
0 suppliers
inquiry

10250-27-8
110 suppliers
$89.00/1g

19312-05-1
9 suppliers
inquiry

10250-27-8
110 suppliers
$89.00/1g

57224-51-8
17 suppliers
$58.00/100 mg

10250-27-8
110 suppliers
$89.00/1g