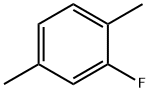
2-Fluoro-p-Xylene synthesis
- Product Name:2-Fluoro-p-Xylene
- CAS Number:696-01-5
- Molecular formula:C8H9F
- Molecular Weight:124.16
95-78-3
265 suppliers
$10.00/1g

696-01-5
75 suppliers
$6.00/1g
Yield: 65.5%
Reaction Conditions:
with sodium nitrite in Et3N-3HF
Steps:
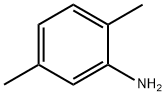
3 Diazotization of 2,5-dimethylaniline in Et3N-3HF
EXAMPLE 3 Diazotization of 2,5-dimethylaniline in Et3N-3HF 2,5-Dimethylaniline (2.3 g, 0.02 mol) was syringed into Et3N-3HF (30 cm3) over a 35 minute period. The reaction mixture turned red during the addition of sodium nitrite (2.0 g, 0.03 mol) and a significant amount of nitrogen was evolved during the reaction. Although a high proportion of sodium nitrite was seen to dissolve a greater proportion of tarry material was formed. The mixture was poured into water (150 cm3) and the remaining contents of the flask washed with further water (30 cm3*2). The mixture was extracted with diethyl ether (150 cm3*2) and the organic layer dried over magnesium sulphate. Fractional removal of diethyl ether afforded an orange oil. Distillation of the oil at 144-146° C. ã atmospheric pressure afforded 1-fluoro-2,5-dimethylbenzene (1.52 g, 65.5%) as a clear colourless liquid. The 19F n.m.r. spectrum had a signal at δF (CDCl3) 121.9, 1-F (ddq, J=8.5 Hz, J=10.5 Hz and J=2.2 Hz). From the GC/MS the compound showed the expected molecular ion at m/z 124 and the expected fragmentation for 1-fluoro-2,5-dimethylbenzene at m/z 109, 101, 96, 83 and 77.
References:
Rhodia Limited US6179970, 2001, B2

89-58-7
130 suppliers
$7.00/5g

696-01-5
75 suppliers
$6.00/1g
106-42-3
393 suppliers
$18.00/25ML

696-01-5
75 suppliers
$6.00/1g

