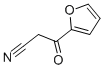
2-FUROYLACETONITRILE synthesis
- Product Name:2-FUROYLACETONITRILE
- CAS Number:31909-58-7
- Molecular formula:C7H5NO2
- Molecular Weight:135.12
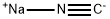
773837-37-9
0 suppliers
inquiry
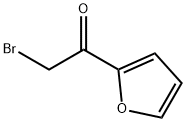
15109-94-1
64 suppliers
$45.00/50 mg

31909-58-7
86 suppliers
$29.19/1g
Yield:-
Reaction Conditions:
Stage #1:sodium cyanide;1-(2-furyl)-2-bromoethanone in ethanol;water for 16 h;
Stage #2: with hydrogenchloride in ethanol;water
Steps:
Preparation of α-Cyanoketones
General procedure: 20 mmol of the appropriate acyl ketone was dissolved in 40 mL of ethyl acetate to make a 0.5 M solution. 2.6 equivalents of CuBr2 was added and the mixture refluxed with a condenser for 3-5 h until the starting ketone was fully consumed as indicated by TLC or 1H NMR. Once the reaction was complete, the mixture was cooled, and then the ethyl acetate was removed under reduced pressure. Hexanes were added to the crude solid and the mixture was sonicated for 5 min. The hexanes were decanted and a fresh volume was added to the remaining solids, again sonicating for 5min. This process was repeated once more, for a total of 3 extractions. The combined hexane layers, which typically appeared as an amber or light yellow solution, were evaporated under reduced pressure to yield the crude product which typically appeared as yellow or orange oil. If a sonicator is not available, a Soxhlet extraction with hexanes gives similar yields. The crude bromoketone oil was next dissolved in a 5:1 mixture of ethanol/water to give a 0.4 M solution overall. The solution was cooled over ice, and then 3 equivalents of NaCN were added. The reaction was stirred overnight (16 h), allowing the ice to melt. The solution was then diluted with enough water to roughly double the initial volume. This solution was filtered through a Celite pad to remove suspended solids. This solution was then acidified by adding concentrated HCl to a stirring solution. CAUTION This will cause the evolution of HCN gas Only perform in a well-ventilated fume hood The acidified solution was allowed to stir for 15 minutes. The solution was checked by pH paper to ensure that it was acidic (pH ≤ 2). If not, additional HCl was added, taking the same precautions noted above. Once the solution was acidic, it was transferred to a separatory funnel and extracted 3x with DCM. The organic layers were combined, dried over magnesium sulfate, filtered, and then evaporated under reduced pressure to obtain the final product. Purity was confirmed by 1H NMR. If significant impurities are observed, the product can be recrystallized, most typically in isopropanol or toluene.
References:
Everson, Nikalet;Yniguez, Kenya;Loop, Lauren;Lazaro, Horacio;Belanger, Briana;Koch, Grant;Bach, Jordan;Manjunath, Aashrita;Schioldager, Ryan;Law, Jarvis;Grabenauer, Megan;Eagon, Scott [Tetrahedron Letters,2019,vol. 60,# 1,p. 72 - 74] Location in patent:supporting information
98-00-0
454 suppliers
$23.00/25mL

75-05-8
963 suppliers
$10.00/10g

31909-58-7
86 suppliers
$29.19/1g

144223-33-6
10 suppliers
$90.00/100mg

75-05-8
963 suppliers
$10.00/10g

31909-58-7
86 suppliers
$29.19/1g
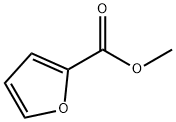
611-13-2
401 suppliers
$13.00/25g

75-05-8
963 suppliers
$10.00/10g

31909-58-7
86 suppliers
$29.19/1g
