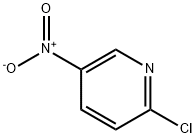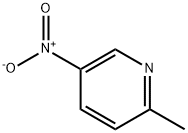
2-Methyl-5-nitropyridine synthesis
- Product Name:2-Methyl-5-nitropyridine
- CAS Number:21203-68-9
- Molecular formula:C6H6N2O2
- Molecular Weight:138.12
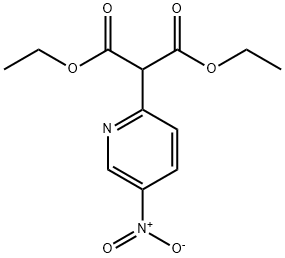
60891-70-5
34 suppliers
inquiry

21203-68-9
250 suppliers
$8.00/1g
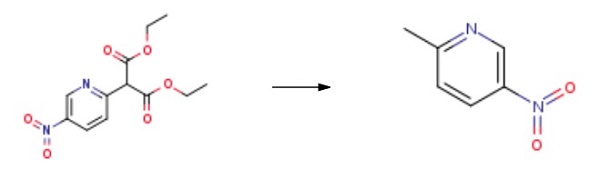
Yield:21203-68-9 83%
Reaction Conditions:
with sulfuric acid;water at 100; for 2 h;
Steps:
28
Reference Example 28: 5-Nitro-2-methyl pyridine. To 2-(5-nitro-pyridin-2-yl)-malonic acid diethyl ester (12.0 g, 42.5 mmol) was added cold aq. 20% sulfuric acid (120 mL) and the mixture was heated to 1000C for 2h. The cooled reaction was added to cold dilute sodium hydroxide solution and the pH adjusted to pH -10. The organics were extracted with dichloromethane (x 4), then the combined organic phases were dried over sodium sulfate. The filtrate was concentrated to afford 2-methyl-5-nitro pyridine (5.0 g, 83 %) as a brown solid.
References:
F2G LTD WO2008/62182, 2008, A1 Location in patent:Page/Page column 117
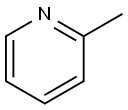
109-06-8
396 suppliers
$16.00/25g

21203-68-9
250 suppliers
$8.00/1g
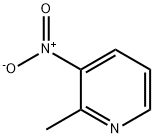
18699-87-1
253 suppliers
$8.00/1g

109-06-8
396 suppliers
$16.00/25g

21203-68-9
250 suppliers
$8.00/1g

14150-94-8
146 suppliers
$17.67/250mgs:
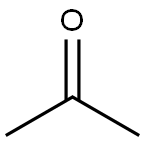
67-64-1
6 suppliers
$17.30/10ml

21203-68-9
250 suppliers
$8.00/1g

100-01-6
10 suppliers
$11.19/25G