2-Thiophenecarbonyl chloride synthesis
- Product Name:2-Thiophenecarbonyl chloride
- CAS Number:5271-67-0
- Molecular formula:C5H3ClOS
- Molecular Weight:146.59
527-72-0
483 suppliers
$5.00/10g

5271-67-0
251 suppliers
$5.00/1g
Yield:5271-67-0 81%
Reaction Conditions:
with thionyl chloride;N,N-dimethyl-formamide;sodium hydroxide in ethyl acetate at 58 - 65; for 2.5 h;Inert atmosphere;
Steps:
14.2 Conversion of 2-Thiophenecarboxylic Acid to 2-Thiophenecarbonyl Chloride
In this experiment, the 2-thiophenecarboxylic acid was the combined solids from Experiments 13.2 and 13.3 of Example 13. [0187] The combined solids of 2-thiophenecarboxylic acid (63.1 g, 493 mmol) was dissolved in ethyl acetate (208 g) in a 3-neck round-bottom flask equipped with a thermocouple, a reflux condenser, and an additional funnel and the system was purged with nitrogen. The reflux condenser outlet was connected to a chilled receiver containing aq. NaOH (20%, 250 g). A catalytic amount of dimethylformamide (DMF) (0.2 mL, 0.005 eq.) was added and the resulting reaction mixture was heated to 65°C with stirring, followed by a slow addition of thionyl chloride (67.2, 565 mmol, 1.15 eq.). During the addition, gases such as sulfur dioxide (S02) and hydrogen chloride (HC1) were released and the reaction temperature dropped to about 58°C. The reaction completed in 2.5 hours with no detectable unreacted 2-thiophenecarboxylic acid by GC/MS.[0188] After cooling, the flask was fitted with a distillation head and 4-Methoxyphenol (8.9 mg) was added as a stabilizer during the distillation. By vacuum distillation (short path), ethyl acetate and thionyl chloride were distilled from the mixture first under a lower vacuum (approximately 60 to 125 mmHg). The remaining mixture was cooled to room temperature and switched to a higher vacuum. Distillation (short path, approximately 4 mmHg) at a vapor temperature of approximately 63°C afforded 2-thiophenecarbonyl chloride as a clear pale yellow oil (56.5 g, 81%). GC-FID confirmed that the obtained material was 2-thiophenecarbonyl chloride with a purity of > 98 area%.
References:
MONSANTO TECHNOLOGY LLC;WALKER, DANIEL PATRICK;MILLER, WILLIAM HAROLD TW2017/36360, 2017, A Location in patent:Paragraph 0190 - 0195
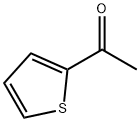
88-15-3
596 suppliers
$5.00/10g

5271-67-0
251 suppliers
$5.00/1g
3437-95-4
247 suppliers
$6.00/5g
122-04-3
8 suppliers
$15.00/1g
636-98-6
16 suppliers
$17.00/5g

5271-67-0
251 suppliers
$5.00/1g