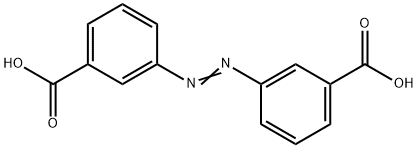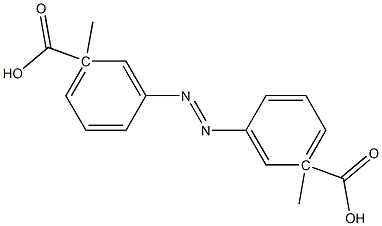
3,3'-Azobisbenzoic acid dimethyl ester synthesis
- Product Name:3,3'-Azobisbenzoic acid dimethyl ester
- CAS Number:53171-92-9
- Molecular formula:C16H14N2O4
- Molecular Weight:298.29
4518-10-9
179 suppliers
$12.80/1g

53171-92-9
0 suppliers
inquiry
Yield:53171-92-9 313.3 mg
Reaction Conditions:
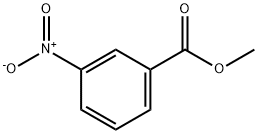
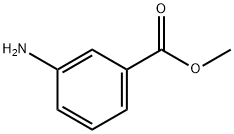
Stage #1: Methyl 3-aminobenzoatewith potassium peroxymonosulfate in dichloromethane;lithium hydroxide monohydrate at 20; for 3 h;
Stage #2: with glacial acetic acid at 20; for 144 h;
Steps:
3 Synthesis of Compound (13)
Commercially available methyl 3-aminobenzoate (hereinafter, also referred to as Compound (12), 500 mg, 1.65 mmol, TCI) was weighed into a 100 ml eggplant flask, dichloromethane (4.5 mL) was added thereto, and the mixture was stirred. Oxone (R) (2028 mg, 3.30 mmol) was weighed into a 100 ml Erlenmeyer flask, water (20 ml) was added thereto, and the mixture was stirred. This aqueous solution was added to the initial solution and stirred vigorously for 3 hours. Thereafter, the mixture was transferred to a separatory funnel to separate an aqueous layer, and the organic layer was washed with 1 N hydrochloric acid, a saturated aqueous sodium bicarbonate solution, water, and saturated saline. Then, sodium sulfate was added thereto, and the organic layer was dried. Sodium sulfate was removed by filtration, and the solvent was distilled off to obtain Compound (13) (orange, solid, 272.4 mg) at a crude yield of 99%. Compound (13) was not further purified, and was used as it was for the synthesis of Compound (14). Synthesis of Compound (14) Methyl 3-aminobenzoate (Compound (12), 205.5 mg, 1.36 mmol) and acetic acid (12.0 ml) were added to the synthesized Compound (13), and the mixture was stirred at room temperature for 6 days. Then, an orange solid was precipitated. This solid was filtered by suction, and washed with water to obtain Compound (14) (orange, solid, 313.3 mg) at a yield of 77% (two-stage yield).
References:
US2022/144757,2022,A1 Location in patent:Paragraph 0227-0229