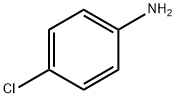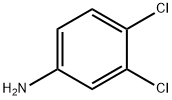
3,4-Dichloroaniline synthesis
- Product Name:3,4-Dichloroaniline
- CAS Number:95-76-1
- Molecular formula:C6H5Cl2N
- Molecular Weight:162.02
Yield:-
Reaction Conditions:
with hydrogen at 90; under 3750.38 - 4500.45 Torr;Temperature;Pressure;
Steps:
1 Example 1 is embodiment cogeneration production of 3,4-dichloroaniline and chloroaniline production process as follows:
chloronitrobenzene chlorine after chlorination of nitrobenzene content 20-35%, 65-80% 3,4-dichloronitrobenzene content of the mixture is washed with water, dehydrated and coke, without the need for solvents case directly through a metering pump it into the hydrogenation reactor, hydrogenation reactor in continuous hydrogenation reaction temperature is controlled at 80 ± 5 , pressure control in 0.5-0.6Mpa, after the hydrogenation reaction vessel through the catalyst filter recovered catalyst can be reused, chloroaniline and 65-80% of 3,4-dichloroaniline mixture, the mixture was watershed (water separation needs to come out before the distillation) after 20-35% of the production, based on the chlorine aniline different boiling 232 and 272 boiling point of 3,4-dichloroaniline, in -0.09Mpa degree continuous vacuum distillation top product purity of 99.5% or more of chloroaniline, column-free recovery of low-chloroaniline after further distillation to obtain a mixture of 3,4-dichloroaniline purity of more than 99.3% of the product.
References:
CN104211601,2016,B Location in patent:Paragraph 0020-0022
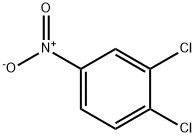
99-54-7
6 suppliers
$13.00/25g

95-76-1
368 suppliers
$10.00/5g
151169-75-4
313 suppliers
$9.00/5g

95-76-1
368 suppliers
$10.00/5g