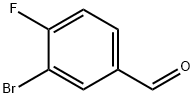
3-Bromo-4-fluorobenzaldehyde synthesis
- Product Name:3-Bromo-4-fluorobenzaldehyde
- CAS Number:77771-02-9
- Molecular formula:C7H4BrFO
- Molecular Weight:203.01
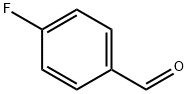
459-57-4
579 suppliers
$6.00/25g

77771-02-9
376 suppliers
$15.00/25 g
Yield:77771-02-9 97%
Reaction Conditions:
with sulfuric acid;sulfur trioxide;bromine;iodine;zinc dibromide at 30 - 40; for 5.91667 h;Product distribution / selectivity;Inert atmosphere;
Steps:
2
Into a 500 mL four necked flask fitted with an overhead stirrer, a condenser and a thermometer pocket, 65% Oleum 204g (7.5 times w.r.t. 4- Fluorobenzaldehyde ) was added to the flask slowly followed by iodine 0.27g (1 wt% w.r.t 4-Fluorobenzaldehyde) addition. The mixture was stirred under N2 atm. for 5 min. Then zinc bromide 0.68g (2.5wt% w.r.t 4- Fluorobenzaldehyde) was added to the reaction mass and the stirring was continued for another 5.0min. Then 4-Fluorobenzaldehyde (27.2g, 0.219mole) was added dropwise over a period of 1.0 h at temperature below 300C. The resulting mixture was stirred for 15 min and then bromine (6.8mL, 0.13 lmol) was added drop wise over a period of 3h at a temperature below 300C. Then the reaction mass was heated to 400C and maintained at same temperature for 90 min. and the reaction was monitored by G.C every 30 min. G.C area% showed 98% product formation. The reaction mass was cooled to 10 0C and was quenched in ice (128.Og) over a period of 2h at a temperature below 25 0C. The organic portion was extracted with 2x 100 mL of toluene followed by washing with 3xl00mL water. The organic layer was treated with thiosulfate to remove unreacted bromine present in the crude. The final mass was washed with water and then the organic layer was separated and dried over Na2SO4 and the liquid was evaporated to give crude pale yellow product 43.19 gm ( 97% yield). GC purity was 96%.
References:
ADITYA BIRLA SCIENCE &;TECHNOLOGY CO. LTD.;MANDAL, Sisir, Kumar;SULEMAN, Inamdur;MORE, Vinod;NIPHADE, Amol WO2010/86877, 2010, A2 Location in patent:Page/Page column 11-12

77771-03-0
133 suppliers
$11.00/1g

77771-02-9
376 suppliers
$15.00/25 g
2973-78-6
214 suppliers
$14.00/5g

77771-02-9
376 suppliers
$15.00/25 g

459-57-4
579 suppliers
$6.00/25g

107-06-2
401 suppliers
$14.00/25g

77771-02-9
376 suppliers
$15.00/25 g