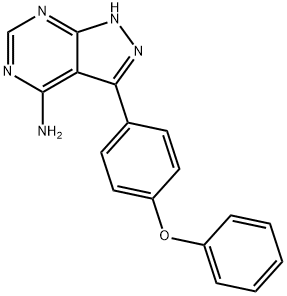
3-(4-Phenoxyphenyl)-1h-pyrazolo[3,4-d]pyrimidin-4-amine synthesis
- Product Name:3-(4-Phenoxyphenyl)-1h-pyrazolo[3,4-d]pyrimidin-4-amine
- CAS Number:330786-24-8
- Molecular formula:C17H13N5O
- Molecular Weight:303.32
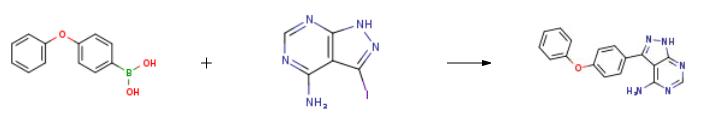
2 g of 3-iodo-4-aminopyrazolo[3,4-d]pyrimidine (7.7 mmol),3.28 g p-phenoxybenzeneboronic acid (15.4 mmol)And 5.28 g of K3PO4 (23.0 mmol) were dissolved in 25 mL of dioxane and 10 mL of water.After stirring for 5-8 minutes, argon gas was passed for 20 minutes.An additional 1.4 g of tetrakis(triphenylphosphine)palladium (1.2 mmol) was added.After heating again for 10 minutes, the heating was started, and the reaction was carried out at 120 ° C for 24 hours.After the reaction was completed, it was cooled to room temperature, stirred for 24 hours to wait for product to precipitate, and the reaction mixture was washed with 25 mL of water and filtered.The filtered solid was again washed with 75 ml of methanol, washed with 50 mL of ethanol, and dried in a dry box.There was obtained 1.75 g of 3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidine-4-amine in a yield of 75%.
330792-70-6
182 suppliers
inquiry

60100-09-6
0 suppliers
inquiry
![3-(4-Phenoxyphenyl)-1h-pyrazolo[3,4-d]pyrimidin-4-amine](/CAS/20150408/GIF/330786-24-8.gif)
330786-24-8
422 suppliers
$55.00/100mg
Yield:330786-24-8 84.5%
Reaction Conditions:
with ammsnium formate at 135; for 15 h;
Steps:
crafting process
Specifically, first add 80.0g of 5-amino-3-(4-phenoxyphenyl)-4-cyano-1H-pyrazole,9.0g of ammonium formate and 600g of formamide were heated to 135°C, and the reaction was stirred at this temperature for 15h, After the reaction was completed, the temperature was lowered to 25° C., 400 g of water was slowly added, the temperature was lowered to 0° C., centrifugation, and 74.2 g of intermediate I was obtained by vacuum drying, yield: 84.5%, HPLC purity 98.0%
References:
CN114853764,2022,A Location in patent:Paragraph 0041-0044
51067-38-0
338 suppliers
$9.00/1g
![3-Iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine](/CAS/GIF/151266-23-8.gif)
151266-23-8
327 suppliers
$22.00/1g
![3-(4-Phenoxyphenyl)-1h-pyrazolo[3,4-d]pyrimidin-4-amine](/CAS/20150408/GIF/330786-24-8.gif)
330786-24-8
422 suppliers
$55.00/100mg

51067-38-0
338 suppliers
$9.00/1g
![7-bromo-2,4,8,9-tetrazabicyclo[4.3.0]nona-2,4,6,9-tetraen-5-amine](/CAS/GIF/83255-86-1.gif)
83255-86-1
199 suppliers
$19.00/5g
![3-(4-Phenoxyphenyl)-1h-pyrazolo[3,4-d]pyrimidin-4-amine](/CAS/20150408/GIF/330786-24-8.gif)
330786-24-8
422 suppliers
$55.00/100mg
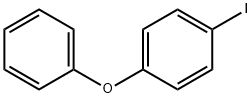
2974-94-9
78 suppliers
$25.00/1g
![4-Aminopyrazolo[3,4-d]pyrimidine](/CAS/GIF/2380-63-4.gif)
2380-63-4
361 suppliers
$9.00/1g
![3-(4-Phenoxyphenyl)-1h-pyrazolo[3,4-d]pyrimidin-4-amine](/CAS/20150408/GIF/330786-24-8.gif)
330786-24-8
422 suppliers
$55.00/100mg
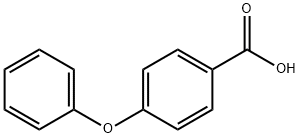
2215-77-2
378 suppliers
$8.00/5g
![3-Iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine](/CAS/GIF/151266-23-8.gif)
151266-23-8
327 suppliers
$22.00/1g
![3-(4-Phenoxyphenyl)-1h-pyrazolo[3,4-d]pyrimidin-4-amine](/CAS/20150408/GIF/330786-24-8.gif)
330786-24-8
422 suppliers
$55.00/100mg