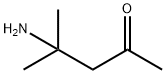
4-AMINO-4-METHYL-2-PENTANONE HYDROGENOXALATE synthesis
- Product Name:4-AMINO-4-METHYL-2-PENTANONE HYDROGENOXALATE
- CAS Number:625-04-7
- Molecular formula:C6H13NO
- Molecular Weight:115.17
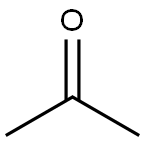
67-64-1
6 suppliers
$17.30/10ml
53422-22-3
2 suppliers
inquiry
63681-01-6
1 suppliers
inquiry
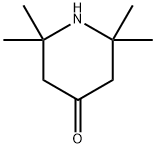
826-36-8
284 suppliers
$6.00/5g
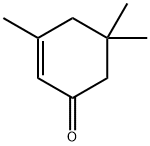
78-59-1
554 suppliers
$11.00/25g
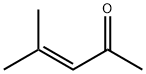
141-79-7
254 suppliers
$10.00/5g
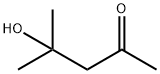
123-42-2
560 suppliers
$19.00/100ml

625-04-7
71 suppliers
$70.00/20mg
Yield: 8.8 %Chromat. , 12.8 %Chromat. , 0.6 %Chromat. , 2.7 %Chromat. , 4.1 %Chromat. , 3 %Chromat. , 0.5 %Chromat.
Reaction Conditions:
with ammonia at 75; under 10501.1 Torr;
Steps:
1. Reaction
Two cylindrical reactors connected in series were filled with Lewatit K2621 (polystyrene catalyst with -SO3- as functional group, from Lanxess) such that this catalyst material was arranged delimited by screens above and below. The bed volume of the catalyst was in each case 600 ml in the moist with water state. The reactor was continuously charged with 630 g/h of acetone and 25 g/h of ammonia. A temperature of 75° C. and a pressure of 14 bar were set. The supply was in total 961.83 g (16.58 mol) of acetone. The LHSV (liquid hourly space velocity, i.e. the volume of reactants added per hour, relative to the reactor volume) was 0.03 to 0.06 h-1 for the ammonia and 0.66 to 1.33 h-1 for the acetone. (0135) 2. I1: Work-Up According to Inventive Method. i.e. with Hydrolysis (0136) 1 kg of the reactor output from the test according to point 1 were mixed with 3 to 5 wt % of water based on the amount of acetone used under point 1, and subsequently distillatively worked up on a rectification column. Distillation was carried out here for sufficient time that all light-boilers (acetone, mesityl oxide, diacetone alcohol, diacetone amine) were removed; TMDH pyridine and TAA and further high-boilers formed were however removed from the bottom as waste products or products of value. Intermediate boilers such as acetonin and phorone were hydrolysed to as great an extent as possible into light-boiling components and were removable as a result. (0137) Thereafter, the proportion of the different components in the bottom and in the distillate were determined by GC [column: HP-50 (30 m), temperature programme: 5° C./min 60-130° C., 15° C./min-270° C., 10 min]. The relative proportions of the respective components in the reactor output, distillate and bottom were determined and this is listed in the following table 1. In the following table 1, the components in the reaction output (with the exception of the water) are listed from left to right according to their boiling points (“NI”=not identified). (0138) The first three rows give the area percentages (including water) of each component in the GC diagram. (0139) The final row gives: (0140) based on the total weight of the bottom product (200 g), the weights of the respective component in the bottom product were determined from the percentage proportion of the respective compound in the GC. The molar amount of the respective component in the bottom product and hence also the molar amount of acetone equivalents corresponding to the respective component can be calculated therefrom. (0141) In this case, acetone corresponds to one acetone equivalent. (0142) In this case, the following compounds correspond to two acetone equivalents: mesityl oxide (and isomer), diacetone alcohol, diacetone amine. (0143) In this case, the following compounds correspond to three acetone equivalents: acetonin, phorone, isophorone. The proportion of acetone equivalents lost in the inventive procedure based on the proportion of acetone used (16.58 mol) was thus determined and was 2.5%.
References:
Evonik Operations GmbH;MINKE, Katharina;Rieb, Julia;Neumann, Manfred US2020/181088, 2020, A1 Location in patent:Paragraph 0133-0144

141-79-7
254 suppliers
$10.00/5g

625-04-7
71 suppliers
$70.00/20mg

67-64-1
6 suppliers
$17.30/10ml

826-36-8
284 suppliers
$6.00/5g

4168-79-0
34 suppliers
$12.00/1mg

123-42-2
560 suppliers
$19.00/100ml
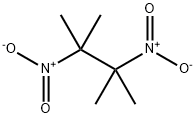
3964-18-9
124 suppliers
$55.00/250mg

625-04-7
71 suppliers
$70.00/20mg
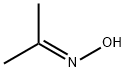
127-06-0
399 suppliers
$5.67/25g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

625-04-7
71 suppliers
$70.00/20mg

67-64-1
6 suppliers
$17.30/10ml

625-04-7
71 suppliers
$70.00/20mg