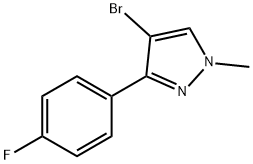
4-BROMO-3-(4-FLUOROPHENYL)-1-METHYL-1H-PYRAZOLE synthesis
- Product Name:4-BROMO-3-(4-FLUOROPHENYL)-1-METHYL-1H-PYRAZOLE
- CAS Number:863605-34-9
- Molecular formula:C10H8BrFN2
- Molecular Weight:255.09
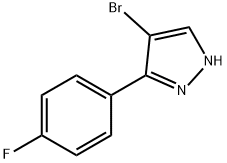
474706-36-0
30 suppliers
$60.00/100mg

74-88-4
341 suppliers
$15.00/10g

863605-34-9
20 suppliers
inquiry
Yield:863605-34-9 54.9%
Reaction Conditions:
Stage #1: 4-bromo-3-(4-fluorophenyl)-1H-pyrazolewith sodium hydride in N,N-dimethyl-formamide;mineral oil at 0 - 20;Inert atmosphere;
Stage #2: methyl iodide in N,N-dimethyl-formamide;mineral oil at 0 - 20;
Steps:
Intermediate 2: 4-bromo-3-(4-fluorophenyl)-1-methyl-1H-pyrazole
To a solution of 4-bromo-3 -(4-fluorophenyl)- lH-pyrazole (14.47 g, 60.0 mmol) in DMF (100 mL) was added 60% NaH dispersion in mineral oil (2.88 g, 72.0 mmol) in portions at 0 °C under nitrogen. The reaction mixture was stirred at rt for 30 min. The reaction mixture was cooled to 0 °C and Mel (4.88 mL, 78 mmol) was added slowly. The reaction mixture was stirred at rt for lh and diluted with EtOAc and water. The layers were separated. The aqueous layer was extracted with EtOAc (2 x 40 mL). The combined organic layer was washed with water and brine, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The crude product was purified by BIOTAGE (300 g Thomson, 50% CH2Cl2/hexane equilibration, 50-80% CH2Cl2/hexane, 3 L, then 80-85% 0.7 L) to give Intermediate 2 (8.4 g, 54.9%) as a white solid. MS(ES): m/z= 254.88/256.88 [M+H]+. HPLC Ret time (Method B): 3.68 min. NMR (400MHz, chloroform-d) δ 7.92 - 7.82 (m, 2H), 7.47 (s, 1H), 7.13 (t, J=8.8 Hz, 2H), 3.94 (s, 3H).
References:
WO2014/100540,2014,A1 Location in patent:Page/Page column 55-56

863605-35-0
2 suppliers
inquiry

863605-34-9
20 suppliers
inquiry

154258-82-9
126 suppliers
$7.00/250mg

863605-34-9
20 suppliers
inquiry
403-42-9
535 suppliers
$5.00/10g

863605-34-9
20 suppliers
inquiry

75175-77-8
48 suppliers
$5.00/250mg

863605-34-9
20 suppliers
inquiry