
4-BROMO-3'-METHOXYBIPHENYL synthesis
- Product Name:4-BROMO-3'-METHOXYBIPHENYL
- CAS Number:74447-69-1
- Molecular formula:C13H11BrO
- Molecular Weight:263.13
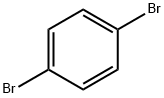
106-37-6
472 suppliers
$9.00/5g
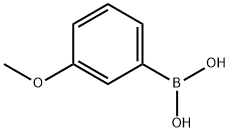
10365-98-7
406 suppliers
$9.00/10g

74447-69-1
27 suppliers
$419.00/1g
Yield:74447-69-1 95%
Reaction Conditions:
with sodium carbonate in ethanol;water at 80; for 0.25 h;Catalytic behavior;Suzuki-Miyaura Coupling;
Steps:
2.4. Typical procedure for the Suzuki-Miyaura cross coupling reaction
General procedure: Aryl halide (1 mmol), phenylboronic acid (1 mmol), Na2CO3(2 mmol) and Fe2O3(at)FLGPd0 nanocatalyst (0.0004 g) were added to a round-bottomed flask containing 3mL of aqueous 50% ethanol(v/v%), and the mixture was stirred at 80 °C for the time listed in Table 2. The progress was monitored by TLC ((n-hexane or n-hexane/EtOAc, 9: 1) or GC. After completion of the reaction, the reaction mixture was cooled to room temperature and the catalyst was separated by an external magnet. Then, the reaction mixture was diluted with water and the resultant mixture extracted with n-hexane to isolate the biphenyl products. The combined organic layers were dried over MgSO4, and the solvent was evaporated under reduced pressure.
References:
Rafiee, Fatemeh;Khavari, Parvaneh;Payami, Zahra;Ansari, Narges [Journal of Organometallic Chemistry,2019,vol. 883,p. 78 - 85]

1003858-50-1
14 suppliers
inquiry

6999-03-7
110 suppliers
$14.00/1g

74447-69-1
27 suppliers
$419.00/1g
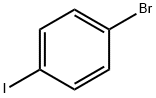
589-87-7
531 suppliers
$10.00/1g
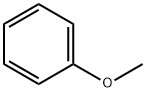
100-66-3
509 suppliers
$10.00/5G

74447-69-1
27 suppliers
$419.00/1g
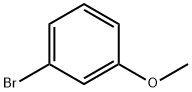
2398-37-0
570 suppliers
$8.00/10g
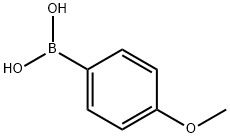
5720-07-0
453 suppliers
$6.00/5g

74447-69-1
27 suppliers
$419.00/1g

589-87-7
531 suppliers
$10.00/1g

10365-98-7
406 suppliers
$9.00/10g

74447-69-1
27 suppliers
$419.00/1g