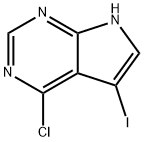
4-Chloro-5-iodo-7H-pyrrol[2,3-d]pyrimidine synthesis
- Product Name:4-Chloro-5-iodo-7H-pyrrol[2,3-d]pyrimidine
- CAS Number:123148-78-7
- Molecular formula:C6H3ClIN3
- Molecular Weight:279.47
Yield:123148-78-7 86.9%
Reaction Conditions:
in N,N-dimethyl-formamide at 20;
Steps:
1.1
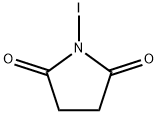
Step 1:4-Chloro-7H-pyrrolo[2,3-d]pyrimidine 10.75 g (70 mmol) and N-iodosuccinimide (16.8 g, 75 mmol) were dissolved in 400 mL of dry DMF and left at ambient temperature in the darkness over night. The solvent was evaporated. The yellow residue was suspended in hot 10% solution of Na2SO3, filtered, washed twice with hot water and crystallized from ethanol to yield 14.6 g (74.6%) of the title compound as off-white crystals. The mother liquid was evaporated up to volume and crystallized again from ethanol to give 2.47 g (12.3%) of the target compound. The total yield is close to 100%;Tm 212-214° C. (dec);UV λmax: 307, 266, 230, 227 nm (methanol);MS: 277.93 (M-H), 313 (M+Cl);1H-NMR (DMSO-d6): 12.94 (s, 1H, NH), 8.58 (s, 1H), 7.94 (s, 1H).
References:
Genelabs Technologies, Inc.;Gilead Sciences, Inc. US2009/48189, 2009, A1 Location in patent:Page/Page column 36
![4-Chloro-7H-pyrrolo[2,3-d]pyrimidine](/CAS/GIF/3680-69-1.gif)
3680-69-1
795 suppliers
$6.00/1g
![4-Chloro-5-iodo-7H-pyrrol[2,3-d]pyrimidine](/CAS/GIF/123148-78-7.gif)
123148-78-7
253 suppliers
$8.00/1g
![Pyrrolo[2,3-d]pyrimidin-4-ol](/CAS/GIF/3680-71-5.gif)
3680-71-5
399 suppliers
$6.00/1g
![4-Chloro-5-iodo-7H-pyrrol[2,3-d]pyrimidine](/CAS/GIF/123148-78-7.gif)
123148-78-7
253 suppliers
$8.00/1g

52133-67-2
183 suppliers
$8.00/1g
![4-Chloro-5-iodo-7H-pyrrol[2,3-d]pyrimidine](/CAS/GIF/123148-78-7.gif)
123148-78-7
253 suppliers
$8.00/1g

7400-05-7
37 suppliers
$60.00/250mg
![4-Chloro-5-iodo-7H-pyrrol[2,3-d]pyrimidine](/CAS/GIF/123148-78-7.gif)
123148-78-7
253 suppliers
$8.00/1g