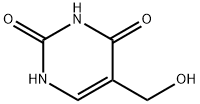
5-Hydroxymethyluracil synthesis
- Product Name:5-Hydroxymethyluracil
- CAS Number:4433-40-3
- Molecular formula:C5H6N2O3
- Molecular Weight:142.11
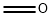
50-00-0
842 suppliers
$10.00/25g
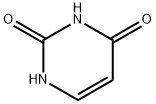
66-22-8
766 suppliers
$5.00/10g

4433-40-3
221 suppliers
$6.00/250mg
Yield:4433-40-3 100%
Reaction Conditions:
with potassium hydroxide in water at 50 - 52; for 68 h;
Steps:
1.a 5-(Hydroxymethyl)-1,3-dihydropyrimidine-2,4-dione
Example 1a 5-(Hydroxymethyl)-1,3-dihydropyrimidine-2,4-dione A 2-L, three-necked flask equipped with a mechanical stirrer, thermometer, condenser, and nitrogen-inlet bubbler was charged with uracil (185.0 g, 1650 mmol) (Aldrich), paraformaldehyde (61.50 g, 2050 mmol as formaldehyde) (Aldrich), and a solution of potassium hydroxide (86.9%, 59.95 g, 928.5 mmol) (Aldrich) in water (1.445 L). The mixture was stirred at 50-52° C. for 68 h. TLC analysis indicated complete reaction. After concentration at 60° C./14 mmHg to a volume of ca. 500 mL, the residue was diluted with acetone (500 mL). The resulting precipitate was collected by filtration, washed with acetone, and dried by suction, then at 50° C./25 mmHg to give crude 5-(hydroxymethyl)-1,3-dihydropyrimidine-2,4-dione (250 g) as a white solid. The combined mother liquor and washes were concentrated to a volume of ca. 100 mL and a solution of hydroxylamine hydrochloride (27.52 g, 396.0 mmol, Aldrich) in water (100 mL) was added. The resulting precipitate was collected by filtration, washed with acetone, and dried by suction to give second crop of crude 5-(hydroxymethyl)-1,3-dihydropyrimidine-2,4-dione (34 g) as a white solid. The two lots were combined (244 g, 4% overweight) and used directly in the next step.
References:
Chen, Yi;Daniewski, Andrzej Robert;Harris, William;Kabat, Marek Michal;Liu, Emily Aijun;Liu, Jin-Jun;Luk, Kin-Chun;Michoud, Christophe US2004/38995, 2004, A1 Location in patent:Page/Page column 4-6

66-22-8
766 suppliers
$5.00/10g

4433-40-3
221 suppliers
$6.00/250mg

65-71-4
502 suppliers
$5.00/5g

4433-40-3
221 suppliers
$6.00/250mg
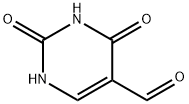
1195-08-0
144 suppliers
$23.00/100mg

89179-86-2
9 suppliers
$502.19/5MG

4433-40-3
221 suppliers
$6.00/250mg

3590-48-5
51 suppliers
$26.00/100 mg
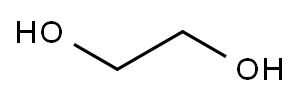
107-21-1
1302 suppliers
$10.00/25g

4433-40-3
221 suppliers
$6.00/250mg

88459-62-5
1 suppliers
inquiry