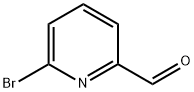
6-Bromopyridine-2-carbaldehyde synthesis
- Product Name:6-Bromopyridine-2-carbaldehyde
- CAS Number:34160-40-2
- Molecular formula:C6H4BrNO
- Molecular Weight:186.01
5315-25-3
457 suppliers
$6.00/5g

34160-40-2
337 suppliers
$6.00/1g
Yield:34160-40-2 62.3 g
Reaction Conditions:
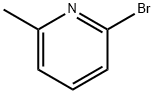
Stage #1:2-bromo-6-methylpyridine with bromine in dichloromethane;water at 10 - 50; for 10 h;
Stage #2: with hexamethylenetetramine in ethanol at 40; for 12 h;
Stage #3: with sulfuric acid;acetic acid in ethanol at 90; for 1 h;
Steps:
1.1; 1.2; 2.1; 2.2; 3.1; 3.2 (1) Preparation of a mixture of compound 2-bromo-6-bromomethylpyridine and 2-bromo-6-(dibromomethyl)pyridine
A 1-L reaction flask was charged with 2-bromo-6-methylpyridine (86 g, 0.5 mol). , 1.0 eq) and dichloromethane (172 mL, 2P), water (172 mL, 2P),The ice water bath was cooled to 10 ° C, stirred rapidly, and liquid bromine (240 g, 1.5 mol, 3.0 eq) was added dropwise, and the temperature was controlled at 10-20 ° C.After the completion of the dropwise addition, the mixture was heated to 50 ° C for 10 hours.Thereafter, it was cooled to room temperature, and the pH was adjusted to 7-8 by adding an aqueous sodium hydrogencarbonate solution.The aqueous phase was separated and extracted with methylene chloride (172 mL, 2P).The organic phase was combined and washed with saturated brine (172 mL, 2P).Drying with anhydrous sodium sulfate, suction filtration and concentration to give a mixture of 110.8 g of 2-bromo-6-bromomethylpyridine and 2-bromo-6-(dibromomethyl)pyridine.The ratio of 2-bromo-6-bromomethylpyridine and 2-bromo-6-(dibromomethyl)pyridine was determined in the liquid phase to be 6:1.(2) Preparation of compound 2-bromo-6-aldehyde pyridinea mixture of 2-bromo-6-bromomethylpyridine and 2-bromo-6-(dibromomethyl)pyridine (110.8 g, theoretical molar amount)0.5 mol, 1 eq), urotropine (140.2 g, 1.0 mol, 2.0 eq) was added to ethanol (435 mL, 4P) and heated to 40 ° C.12 hours.Thereafter, it was cooled to room temperature with acetic acid (220 mL, 2P), concentrated sulfuric acid (15 mL, 0.137 P), and then heated to 90 ° C.After an hour, it was filtered while cooling to 5 ° C, and the filter cake was washed with isopropyl ether to give 75.3 g of a yellow solid. The solid plus ethanol (75.3 mL) / water(8.37mL) recrystallization, to obtain 62.3g white solid, melting point 78.9-80.2 ° C, liquid phase 99.0%, two-step total yield67%.
References:
Anhui Haofan Biological Co., Ltd.;Wang Guichun;Lv Minjie;Liu Min CN109879815, 2019, A Location in patent:Paragraph 0058-0086
33674-96-3
263 suppliers
$8.00/250mg

34160-40-2
337 suppliers
$6.00/1g
626-05-1
498 suppliers
$9.00/10g

34160-40-2
337 suppliers
$6.00/1g

626-05-1
498 suppliers
$9.00/10g
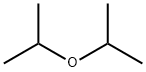
108-20-3
542 suppliers
$25.00/25mL

34160-40-2
337 suppliers
$6.00/1g

82315-66-0
12 suppliers
inquiry

34160-40-2
337 suppliers
$6.00/1g