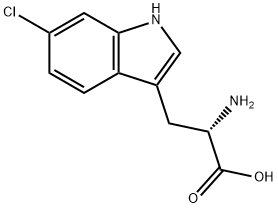
6-CHLORO-L-TRYPTOPHAN synthesis
- Product Name:6-CHLORO-L-TRYPTOPHAN
- CAS Number:33468-35-8
- Molecular formula:C11H11ClN2O2
- Molecular Weight:238.67
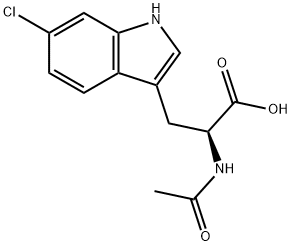
50517-10-7
16 suppliers
$110.50/50mg

33468-35-8
115 suppliers
$65.00/25mg
Yield:33468-35-8 43%
Reaction Conditions:
Stage #1: Nα-acetyl-6-chloro-D,L-tryptophanwith Acylase I;cobalt(II) chloride hexahydrate in aq. phosphate buffer at 37; for 24 h;Enzymatic reaction;
Stage #2: with lithium hydroxide at 60; pH=8; for 0.0833333 h;
Steps:
This compound was synthesized according to the synthetic scheme described in FIGURE 6. To the solution 6-chloroindole (500 mg, 3.31 mmol, I eq) in acetic acid (10 mL) L-serine (695 mg, 6.62 mmol, 2 eq) and acetic anhydride (3.1 mL, 33.1 mmol, 10 eq) were added and the mixture was stirred under Ar at 73°C for 4 h. The reaction mixture was concentrated to half of the volume, diluted with water and extracted with EtOAc. The organic layers were combined, dried over sodium sulfate and evaporated to yield crude product N°-acetyl-6-chloro-D,L-tryptophan 686 mg, 74% yield). 111 NMR (500 MHz, CD3OD) 81.91 (s, 3H), 3.15-3.3 1 (m, 2H), 4.67 (t, IH), 6.97 (dd, IH), 7.10 (s, 1H), 7.33 (d, IH), 7.53 (d, IH). The A°-acetyl-6-chloro-D,L-tryptophan (686 mg, 145 mmol) was dissolved in phosphate buffer (50 mL) containing 1 mM CoCl26H2O and pH 8.0. To the reaction mixture acylase I was added (500 mg) and the reaction was stirred at 37°C for 24 h with occasional adjustment of pH to 8.0 with LIOH. The reaction mixture was heated to 60°C for 5 mm, cooled to room temperature and filtered through celite. The filtrate was acidified to pH around 3 using HCI and extracted with EtOAc. The aqueous layer was lyophilized and used as crude product for the next reaction. Estimated yield of 43% (based on theoretical yield for the Lenantiomer), 125.4 mg. 6-chloro-L-tryptophan (125.4 mg, 0.527 mmol, 1 eq) was dissolved in a water/acetone mixture (1:1, v/v) to which NaHCO3 (88.5 rng, 1.05 mmol, 2 eq) was added. Fmoc-OSu (195.6 rng, 0.58 mmol, 1.1 eq) was dissolved in acetone and added to the reaction mixture portion wise over the course of 3 h. Following reaction completion the acetone was evaporated and the aqueous layer acidified with acetic acid to pH about 3 followed by EtOAc extraction. The organic layers were combined, dried over sodium sulfate and evaporated. The crude product was purified with flash column chromatography and solvent system hexanes:EtOAc:AcOH (10:9:1) to yield pure Fmoc-6-chloro-L-tryptophan (237 mg, 98%). ‘H NMR (500 MHz, CD3OD), 83.16-3.32 (m, 211), 4.65 (t, 1H), 6.98 (dd, 1H), 7.12 (s, 1H), 7.3- 7.55 (m, 8H), 7.79 (d, 211). MS (ESI) calculated for C26H21C1N204 [M+Hj: m/z 460.12, found460.4.
References:
WO2015/153761,2015,A2 Location in patent:Paragraph 00256

17422-33-2
312 suppliers
$10.00/1g
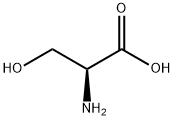
56-45-1
738 suppliers
$5.00/25g

33468-35-8
115 suppliers
$65.00/25mg

769973-16-2
0 suppliers
inquiry

33468-35-8
115 suppliers
$65.00/25mg
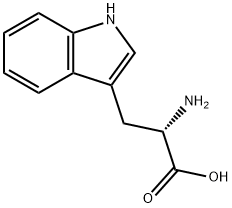
73-22-3
945 suppliers
$5.00/250mg

33468-35-8
115 suppliers
$65.00/25mg

5446-05-9
77 suppliers
$6.00/250mg

33468-35-8
115 suppliers
$65.00/25mg