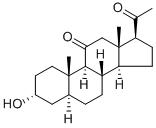
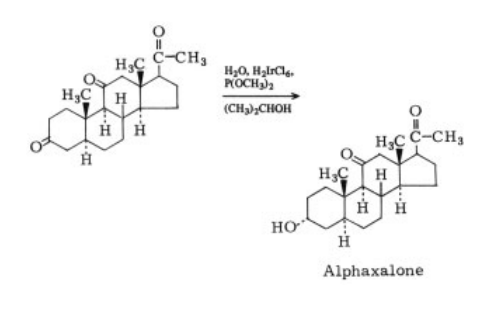
Alfaxalone synthesis
- Product Name:Alfaxalone
- CAS Number:23930-19-0
- Molecular formula:C21H32O3
- Molecular Weight:332.48
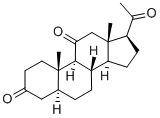
2089-06-7
17 suppliers
inquiry

23930-19-0
65 suppliers
$165.00/2.5mg
Yield:-
Reaction Conditions:
with (+)-β-chloro diisopinocampheyl borane in tetrahydrofuran at 0 - 20; for 3 h;
Steps:
3 Example 3: Synthesis of Stage C/ Formula II:
Arranged clean and dry 4-neck RBF. Charged 1500mL Tetrahydrofuran at RT. Charged lOOg compound of Stage B/ Formula III at RT. The reaction mixture was stirred at RT. The reaction mixture was cooled 0° to -10°C. Slowly added 300mL (-) DIP chloride at 0° to - 10°C. The reaction mixture was stirred for 3.0 hrs at 0° to -10°C. Slowly added 50mL methanol. The reaction mixture was stirred for lhr. Slowly added acetic acid solution (52mL acetic acid in 100ml water). The reaction mixture was stirred for 1.0 hr. The organic layer was separated. Organic layer was washed with potassium carbonate solution (125g potassium carbonate in 500mL water). The reaction mixture was stirred for 1.0 hr. Organic layer was separated. Organic layer was washed with sodium chloride solution (25g sodium chloride in 500mL water). The reaction mixture was stirred for 1.0 hrs. Organic layer was separated. Distilled out organic layer u/v at 50°C strip out with 50mL Heptane. Obtained oily mass 225-250g. Charged 1000 mL DCM to the oily mass. The reaction mixture was stirred for 15 min. Organic layer was washed with Sodium bicarbonate solution (1000 mL 10% aq. sodium bicarbonate solution). The reaction mixture was stirred for 1.0 hr. The organic layer was separated. Organic layer was washed with sodium chloride solution (25g sodium chloride in 500mL water). The reaction mixture was stirred for 1.0 hr. Organic layer was separated. Distilled out organic layer u/v at 40°C and strip out with 50mL heptane. Charged 1000 mL heptane to above reaction mass. The reaction mixture was stirred for 10 hr at 25-30°C. The reaction mixture was filtered and washed with lOOmL heptane. The solid obtained was dried at 50°C for 8 hr to obtain Stage C / Formula II. Dry wt. = 64- 68g.
References:
WO2022/195627,2022,A1 Location in patent:Page/Page column 13
![Pregnane-11,20-dione, 3-[(methylsulfonyl)oxy]-, (3β,5α)-](/CAS/20210305/GIF/1426309-72-9.gif)
1426309-72-9
0 suppliers
inquiry

23930-19-0
65 suppliers
$165.00/2.5mg

3684-81-9
0 suppliers
inquiry

23930-19-0
65 suppliers
$165.00/2.5mg

81800-93-3
0 suppliers
inquiry

23930-19-0
65 suppliers
$165.00/2.5mg

38368-22-8
0 suppliers
inquiry

23930-19-0
65 suppliers
$165.00/2.5mg