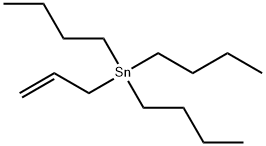
Allyltributyltin synthesis
- Product Name:Allyltributyltin
- CAS Number:24850-33-7
- Molecular formula:C15H32Sn
- Molecular Weight:331.12
Yield:24850-33-7 96%
Reaction Conditions:
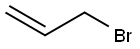
Stage #1: allyl bromidewith iodine;magnesium in diethyl ether; for 1.5 h;Inert atmosphere;Reflux;
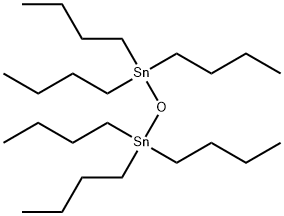
Stage #2: tributyltin oxide in diethyl ether at 20 - 38;Inert atmosphere;Reflux;Concentration;
Steps:
3.3 (3) Synthesis of allyl tributyltin
Equipped with a dry three-necked flask with mechanical stirring was added magnesium turnings 7.2g (0.30mol) and 100mL of anhydrous ether and a smallamount of iodine, a dropping funnel was charged with allyl bromide 21.78g (0.18mol) and 15mL anhydrous In the ether, the whole system was protected with nitrogen, and thetemperature of the system was gradually increased after the dropwise addition of the allyl bromide solution from 1 to 1.2 mL. The reaction was initiated.The remaining allylbromide solution wasadded dropwise, keeping the system slowly refluxing.The mixture was refluxed for 1.5 hours after completion of the addition. Heating was then stopped and a mixture of 15 mL of dry diethylether and tributyltin oxide 19 g (0.062 mol)was addedand the reaction was maintained at a reaction temperature of 36-38C.After the addition was completed, thereaction mixture was further refluxed for 1.5 hours, then stirred at room temperature overnight, then saturated aqueous ammonium chloride solution was added, and the mixture wasextractedwith n-hexane three times.The organic layer was dried over anhydrous magnesium sulfate and filtered. The residual oil, after removal of most of the solvent, was distilled under reduced pressure. Fraction 19.7 g (96%) was collected as a colorless oil.
References:
CN107746396,2018,A Location in patent:Paragraph 0014; 0019-0020; 0026; 0032
106-95-6
416 suppliers
$10.00/5g
56-35-9
183 suppliers
$30.00/100g

24850-33-7
183 suppliers
$12.00/5g
1461-22-9
204 suppliers
$10.00/10g

106-95-6
416 suppliers
$10.00/5g

24850-33-7
183 suppliers
$12.00/5g

1461-22-9
204 suppliers
$10.00/10g
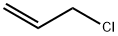
107-05-1
398 suppliers
$16.80/100ML

24850-33-7
183 suppliers
$12.00/5g

1461-22-9
204 suppliers
$10.00/10g
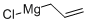
2622-05-1
152 suppliers
$40.00/500mg

24850-33-7
183 suppliers
$12.00/5g
