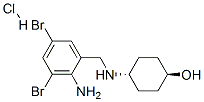
Ambroxol hydrochloride synthesis
- Product Name:Ambroxol hydrochloride
- CAS Number:23828-92-4
- Molecular formula:C13H19Br2ClN2O
- Molecular Weight:414.56
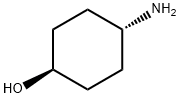
27489-62-9
453 suppliers
$5.00/5g
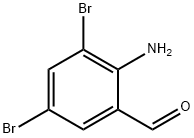
50910-55-9
523 suppliers
$6.00/5g

23828-92-4
568 suppliers
$5.00/50mg
Yield:23828-92-4 96.26%
Reaction Conditions:
Stage #1:trans-4-hydroxycyclohexylamine;3,5-dibromo-2-amino benzaldehyde with lithium perchlorate;sodium tris(acetoxy)borohydride in 1,2-dichloro-ethane at 20; for 1.5 h;
Stage #2: with hydrogenchloride in water;acetone at 20; for 1 h;Solvent;
Steps:
13-17 Example 13: Synthesis of Ambroxol Hydrochloride
In the reaction flask, add 20mmol of trans-4-aminocyclohexanol, 23.2mmol of compound 2, 0.8mmol LiClO4, and 22mmol of NaBH(OAc)3, then add 100mL of 1,2-dichloroethane, and stir for 1.5h at room temperature. . After the reaction, the reaction solution was poured into 100 mL of ice water, and extracted with 100 mL of dichloromethane to obtain an oil phase.The solvent was removed by rotary evaporation under reduced pressure to obtain a yellow liquid, which was dissolved in 100 mL of acetone, 5 mL of concentrated hydrochloric acid was added dropwise under stirring at room temperature, a light yellow precipitate appeared, stirred at room temperature for 1 h, filtered, and washed with acetone to obtain a light yellow crude product. It is recrystallized with water, decolorized by activated carbon, and dried to obtain 4.666 g of white ambroxol hydrochloride with a yield of 96.26% and a purity of 99.76%.
References:
Shandong Luoxin Pharmaceutical Group Hengxin Pharmaceutical Co., Ltd.;Shandong Luoxin Pharmaceutical Group Co., Ltd.;Shandong Yuxin Pharmaceutical Co., Ltd.;Sun Song;Cao Chuanzhen;Zhou Yan;Zhu Yuqing;Xu Guichao CN111072500, 2020, A Location in patent:Paragraph 0058-0067; 0076; 0077

54840-15-2
105 suppliers
$8.00/1g

23828-92-4
568 suppliers
$5.00/50mg

111300-06-2
183 suppliers
$6.00/1g

23828-92-4
568 suppliers
$5.00/50mg
123-30-8
605 suppliers
$10.00/5g

23828-92-4
568 suppliers
$5.00/50mg
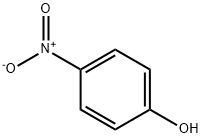
100-02-7
5 suppliers
$11.00/5G

23828-92-4
568 suppliers
$5.00/50mg