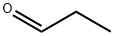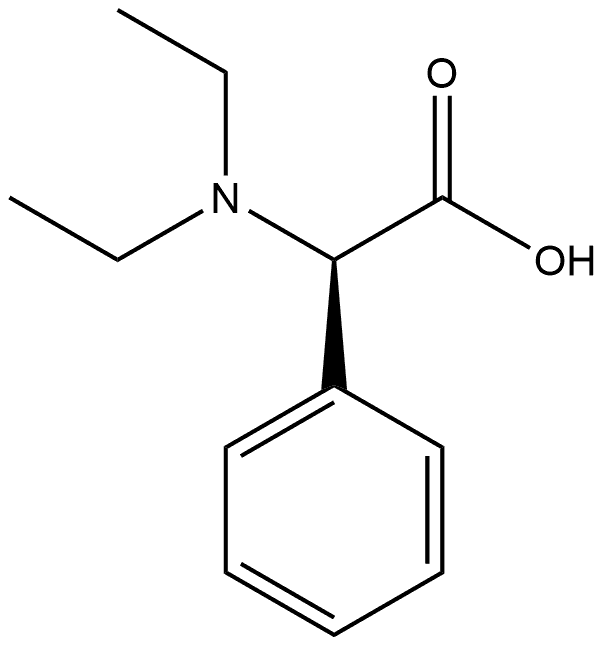
Benzeneacetic acid, α-(diethylamino)-, (αR)- synthesis
- Product Name:Benzeneacetic acid, α-(diethylamino)-, (αR)-
- CAS Number:1007912-96-0
- Molecular formula:C12H17NO2
- Molecular Weight:207.27
Yield:1007912-96-0 61%
Reaction Conditions:
with hydrogenchloride;sodium cyanoborohydride in methanol;water;
Steps:
14 Preparation 14
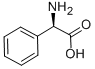
Preparation 14 (R)-Diethylamino-phenyl-acetic acid Sodium cyanoborohydride (15 g, 238 mmol) was added in portions over a few minutes to a cooled (ice/water) mixture of (R)-amino-phenyl-acetic acid (6.00 g, 39.7 mmol) and methanol (150 mL), and stirred for 5 min. Acetaldehyde (40 mL) was added drop-wise over 10 min. The reaction mixture was stirred at the cooled temperature for 45 min and at ambient temperature for 6.5 hr. The reaction mixture was again cooled to 0° C. Additional acetaldehyde (60 mL) was then added drop-wise over 10 min. The reaction mixture was stirred at 0° C. for 45 min and at ambient temperature overnight. The reaction mixture was cooled with an ice-water bath and treated with water (3 mL). Concentrated HCl was added dropwise over 45 min until the pH of the mixture was 1.5-2.0. The cooling bath was removed and stirring was continued while adding concentrated HCl to maintain the pH of the mixture ~1.5-2.0. The reaction mixture was stirred overnight, filtered, and the filtrate was concentrated under vacuum. The crude material was purified by preparative HPLC and washed with ethyl acetate to afford the title compound as a shiny white solid (5 g, 61% yield). 1H NMR (d6-DMSO, 400 MHz) δ (ppm) 7.47 (m, 2H), 7.36 (m, 3H), 4.34 (s, 1H), 2.90 (m, 2H), 2.86 (m, 2H), 1.03 (t, 6H).
References:
US2012/114600,2012,A1