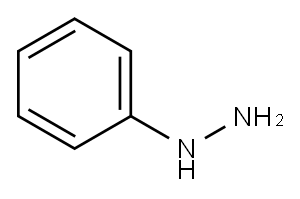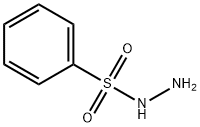
Benzenesulfonyl hydrazide synthesis
- Product Name:Benzenesulfonyl hydrazide
- CAS Number:80-17-1
- Molecular formula:C6H8N2O2S
- Molecular Weight:172.2
ArSO2NHNH2 +HNO2→RSO2N3 +2H2O
98-09-9
341 suppliers
$12.00/25 g

80-17-1
154 suppliers
$23.00/25g
Yield:80-17-1 90%
Reaction Conditions:
with hydrazine hydrate in tetrahydrofuran at -8; for 0.5 h;
Steps:
General Procedure for Sulfonylhydrazide Synthesis (2a-h)
General procedure: To a solution of 80% hydrazine hydrate (0.1 ml, 2.1 mmol, 2.1 eq) in THF (10 ml) was slowly added sulfonylchloride (1 mmol, 1 eq). The mixture was stirred at -8°C for 30 min. The mixture was extracted with ethyl acetate, and the filtrate was concentrated under reduced pressure. The residue was purified by column chromatography on silica gel 60 (Merck; CHCl3/CH3OH, 9.4:0.6) to give sulfonylhydrazides. Benzenesulfonohydrazide (2a) (yield, 90%) Melting point, 102-104°C. 1H NMR (300 MHz, CDCl3, δ= ppm): 3.62 (2H, s), 5.62 (1H, s), 7.57 (2H, t, J = 7.3 Hz), 7.65 (1H, t, J = 7.3 Hz), 7.93 (2H, d, J = 7.0 Hz). 13C NMR (75 MHz, CDCl3, δ = ppm): 128.2, 129.3, 133.5, 136.3.
References:
Fernandes, Thais Batista;de Azevedo, Ricardo Alexandre;Yang, Rosania;Teixeira, Sarah Fernandes;Trossini, Gustavo Henrique Goulart;Barbuto, José Alexandre Marzagão;Ferreira, Adilson Kleber;Parise-Filho, Roberto [Letters in drug design and discovery,2018,vol. 15,# 12,p. 1288 - 1298]

46248-01-5
42 suppliers
$45.00/10mg

80-17-1
154 suppliers
$23.00/25g
98-11-3
363 suppliers
$19.50/100g

80-17-1
154 suppliers
$23.00/25g

80-18-2
285 suppliers
$5.00/5g

80-17-1
154 suppliers
$23.00/25g