carbon monoxide synthesis
- Product Name:carbon monoxide
- CAS Number:201230-82-2
- Molecular formula:
- Molecular Weight:0
Yield: 80% , 6%
Reaction Conditions:
with oxygen;(x)H2O*NO3(1-)*AlCo0005H6.08Mg2Ni0035O6.08(1+) at 900;Catalytic behavior;Reagent/catalyst;Temperature;
Steps:
General procedure: The catalytic properties of the samples in POM andDRM were studied in a heated flow-type quartz reactorwith a thermocouple pocket. The end of the thermocouplewas located in the middle of the catalystbed. The catalyst precursor powders were pelletizedand ground to select a fraction with a grain size of 0.5-1 mm. The catalyst (weight, 0.2 g; bed height, 1 mm)was placed on a quartz fiber substrate. For the POMreaction, the free volume of the reactor was filled withquartz chips. The reactor was fed with CH4-O2 orCH4-CO2 mixtures undiluted with an inert gas (manufacturedat OAO Moscow Gas Refining Plant; purityof 99.9%) at ratios of CH4/O2 = 2 and CH4/CO2 = 1and a space velocity of 11-12 and 15-16 L/(gcat h),respectively; the catalyst was heated to a predeterminedtemperature. After analysis, the temperaturewas adjusted to predetermined values. Product compositionwas analyzed by gas chromatography asdescribed in [5, 8].
References:
Baranchikov, A. E.;Bykov, M. A.;Danilov, V. P.;Dedov, A. G.;Krasnobaeva, O. N.;Loktev, A. S.;Moiseev, I. I.;Mukhin, I. E.;Nosova, T. A.;Yorov, Kh. E. [Petroleum Chemistry,2020,vol. 60,# 2,p. 194 - 203][Neftekhimiya,2020,vol. 60,# 2,p. 214 - 224,11]
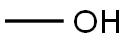
67-56-1
732 suppliers
$7.29/5ml-f
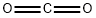
124-38-9
122 suppliers
$175.00/23402
201230-82-2
1 suppliers
inquiry

1333-74-0
112 suppliers
$190.00/56L
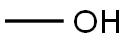
67-56-1
732 suppliers
$7.29/5ml-f
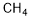
34557-54-5
0 suppliers
inquiry
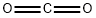
124-38-9
122 suppliers
$175.00/23402
201230-82-2
1 suppliers
inquiry

1333-74-0
112 suppliers
$190.00/56L

31588-18-8
27 suppliers
$181.00/500mg

579-93-1
54 suppliers
$22.00/250mg
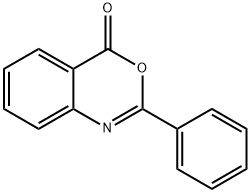
1022-46-4
70 suppliers
$90.00/25mg
201230-82-2
1 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
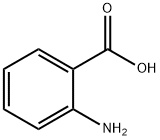
118-92-3
4 suppliers
$21.60/25g
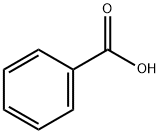
65-85-0
1324 suppliers
$10.00/25 g