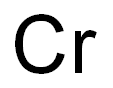
Chromium synthesis
- Product Name:Chromium
- CAS Number:7440-47-3
- Molecular formula:Cr
- Molecular Weight:52
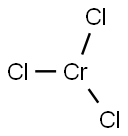
10025-73-7
203 suppliers
$22.39/5G
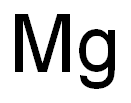
7439-95-4
8 suppliers
$11.60/0.9g
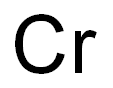
7440-47-3
247 suppliers
$22.00/100g
Yield:7440-47-3 94.2%
Reaction Conditions:
with potassium chloride;sodium chloridebyproducts: MgCl2; 12 kg CrCl3, 3.5 kg Mg, 17.6 kg KCl and 12.4 kg NaCl mixed then heated in electric furnace at 800°C; formed MgCl2 dissolved and filtrated, remainder washed with water twice then with dild. HNO3;
References:
[Gmelin Handbuch der Anorganischen Chemie,Gmelin Handbook: Cr: MVol.A1, 5.2, page 228 - 229] I. G. Farbenindustrie A.-G. DE589987, 1933, C [C.,1934,vol. I,p. 3650][C.,1934,vol. I,p. 3650]

10025-73-7
203 suppliers
$22.39/5G
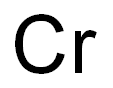
7440-47-3
247 suppliers
$22.00/100g