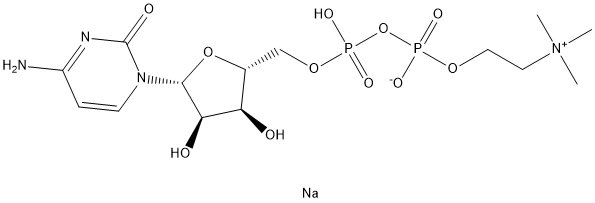
Citicoline sodium synthesis
- Product Name:Citicoline sodium
- CAS Number:33818-15-4
- Molecular formula:C14H26N4O11P2.Na
- Molecular Weight:511.31
987-78-0
397 suppliers
$8.00/100mg

33818-15-4
465 suppliers
$21.00/1g
Yield:33818-15-4 115 mg
Reaction Conditions:
with sodium 2,2,2-trifluoroacetate;triethylamine carbonateInert atmosphere;
Steps:
1-(b-D-Ribofuranosyl)cytosine-50-diphosphate choline (5)
A stirred suspensionof the free acid of CMP (100 mg, 0.31 mmol) in DMF (2 mL) and tributylamine(150 lL, 0.62 mmol) was heated for 10 min at 50 °C and allowed to cool at room temperature. Then, CDI (250 mg, 1.55 mmol) was added and the suspension turned to a clear solution after a few minutes. The anhydrous reaction mixture was stirred for 30 min at room temperature and treated with anhydrous methanol (100 lL, 2.48 mmol) to hydrolyze the CDI excess. After 30 min, anhydrous zinc chloride (59 mg, 0.43 mmol) was added followed by a solution of choline phosphate (0.77 M solution in anhydrous DMF, 2 mL,1.55 mmol). The reaction mixture was stirred for 24 h at room temperature. After evaporation of the solvents in vacuo, CDP-Cho 5 was puried by anion-exchange chromatography. The residue dissolved in waterwas puried bySephadex DEAE A-25 resin ion exchange column chromatography with a linear gradient 0-0.5 M of ammonium bicarbonate, followed by chromatography on RP-18. The triethylammonium counter ions were exchanged to sodium by passing the nucleotide solution through a Dowex 50WX8 column. 1-(b-D-ribofuranosyl)cytosine-5'-diphosphate choline (sodium salt) was obtained as a white solid after freeze-drying (115 mg, 74% yield). HPLC: tR = 8.8 min (kmax276 nm); 1H NMR (400 MHz, D2O) d 7.86 (d, 1H, H6, J6-5 = 7.6 Hz), 6.04 (d, 1H,H5, J5-6 = 7.6 Hz), 5.92 (d, 1H, H10 , J10 -20 = 4.0 Hz), 4.31 (sl, 2H, CH2-O), 4.28-4.08(m, 5H, H20 H30 H40 H50 a H50 b), 3.60 (t, 2H, CH2-N, J = 4.2 Hz), 3.14 (s, 9H, CH3); 13CNMR (101 MHz, D2O) d 166.2 (s, C4), 157.7 (s, C2), 141.4 (s, C6), 96.6 (s, C5),89.3 (s, C10), 82.6 (d, C40, JC-P = 8.9 Hz), 74.1 (s, C20), 69.3 (s, C30), 65.9 (s, CH2N),64.7 (d, C50, JC-P = 5.1 Hz), 59.9 (s, CH2O), 53.9 (s, CH3); 31P NMR (162 MHz, D2O) d 11.35 (d, 2Jab = 21.4 Hz), 12.14 (d, 2Jab = 21.4 Hz); HRMS (ESI) m/z:[M+H]+ Calcd for C14H26N4O11NaP2 511.0971. Found 511.0973.
References:
Ghezal, Salma;Thomasson, Maggie S.;Lefebvre-Tournier, Isabelle;Périgaud, Christian;Macnaughtan, Megan A.;Roy, Béatrice [Tetrahedron Letters,2014,vol. 55,# 38,p. 5306 - 5310] Location in patent:supporting information

63-37-6
542 suppliers
$12.00/25g

4826-71-5
159 suppliers
$8.00/5g

33818-15-4
465 suppliers
$21.00/1g
1310-73-2
1347 suppliers
$14.00/25g

33818-15-4
465 suppliers
$21.00/1g

