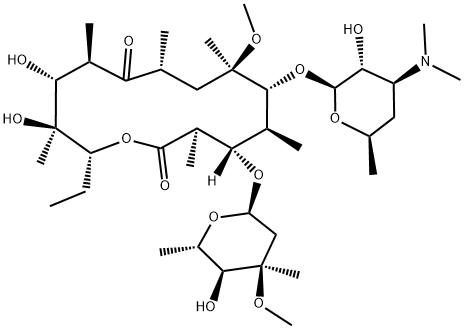
Clarithromycin synthesis
- Product Name:Clarithromycin
- CAS Number:81103-11-9
- Molecular formula:C38H69NO13
- Molecular Weight:747.95
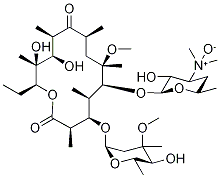
118074-07-0
58 suppliers
$71.00/10mg

81103-11-9
699 suppliers
$5.00/100mg
Yield:81103-11-9 96%
Reaction Conditions:
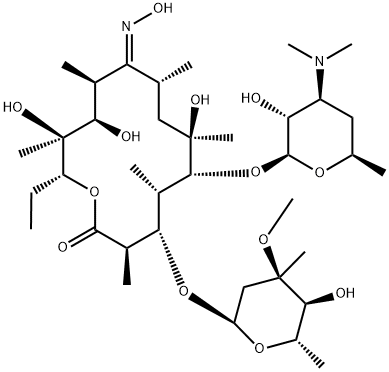
Stage #1: 6-O-methylerythromycin A N-oxidewith tin(ll) chloride in isopropyl alcohol at 30 - 40; for 2 h;
Stage #2: with sodium hydrogencarbonate in water;isopropyl alcohol;
Steps:
3 Example 3; Preparation of clarithromycin by using stannous Chloride Dihydrate as a Reducing Agent
Example 3; Preparation of Clarithromycin by using Stannous Chloride Dihydrate as a Reducing Agent; [00051] 3.82 g(5 mmol) of 6-O-methylerythromycin A N-oxide obtained in Example 1 was suspended in 30 ml of isopropanol. Added thereto was 2.26 g(2.0 mmol) of stannous chloride dihydrate and the mixture was stirred at a temperature ranging from 30 to 40° C. for 2 hours. A saturated sodium bicarbonate solution was added to the resulting mixture and extracted twice with ethylacetate. The combined organic layer was washed with water, dried over anhydrous magnesium sulfate, and then, concentrated under a reduced pressure to obtain 3.60 g of clarithromycin as a white powder in a yield of 96%. [00052] mp: 220223° C.(mp in literature: 222225° C.). [00053] 1H-NMR (CDCl3, ppm): δ 5.06(dd, 1H, 13-H), 4.92(d, 1H, 1-H), 4.44(d, 1H, 1'-H), 4.02(dq, 1H, 5-H), 3.78(dd, 1H, 3-H), 3.77(d, 1H, 11-H), 3.67(d, 1H, 5-H), 3.57(ddq, 1H, 5'-H), 3.33(s, 3H, 3-OCH3), 3.20(dd, 1H, 2'-H), 3.072.95(m, 2H, 10-H and 4-H), 3.03(s, 3H, 6-OCH3), 2.87(dq, 1H, 2-H), 2.58(ddq, 1H, 8-H), 2.40(ddd, 1H, 3'-H), 2.37(d, 1H, 2-Heq), 2.28(s, 6H, 3'-N(CH3)2), 2.001.80(m, 3H, 4-H and 7-H2), 1.41(s, 3H, 18-H), 1.13(s, 3H, 6-CH3), 0.85(t, 3H, 14-CH3).
References:
US6809188,2004,B1 Location in patent:Page column 6; 7
50-00-0
842 suppliers
$10.00/25g

118074-07-0
58 suppliers
$71.00/10mg

81103-11-9
699 suppliers
$5.00/100mg
13127-18-9
193 suppliers
$49.00/100mg

74-88-4
341 suppliers
$15.00/10g

81103-11-9
699 suppliers
$5.00/100mg

930287-49-3
0 suppliers
inquiry

81103-11-9
699 suppliers
$5.00/100mg
791845-56-2
0 suppliers
inquiry

81103-11-9
699 suppliers
$5.00/100mg