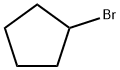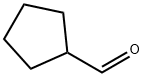
Cyclopentanecarbaldehyde synthesis
- Product Name:Cyclopentanecarbaldehyde
- CAS Number:872-53-7
- Molecular formula:C6H10O
- Molecular Weight:98.14

38389-61-6
0 suppliers
inquiry

872-53-7
256 suppliers
$9.00/1g
Yield:872-53-7 71 %Spectr.
Reaction Conditions:
Stage #1: 6-iodo-1-ethoxyhexyne in hexanes;diethyl ether at -78 - 20; for 2.66667 h;Inert atmosphere;
Stage #2: with methanol in hexanes;diethyl ether at -78 - 20; for 0.5 h;
Stage #3: with perchloric acid in hexanes;diethyl ether at 20; for 18 h;regiospecific reaction;
Steps:
4.8. Hydrolysis of 2a and 2b. Formation of cyclopentane carboxaldehyde
General procedure: A 0.1 M solution of 0.27 mmol iodo ynol ether (2a or 2b) in hexanes-Et2O was deoxygenated by bubbling N2 for 5 min. The mixture was cooled to -78 °C. A solution of freshly titrated n-BuLi in hexanes (0.57 mmol) was added dropwise. The solution was stirred for 10 more minutes at -78 °C and then the cooling bath was removed. The reaction mixture was stirred 2 h at room temperature in order for the cyclization to occur. MeOH (6 equiv) was added and the reaction mixture was stirred for another 30 min at room temperature. To this solution were added 1-2 drops of 35% perchloric acid. The reaction mixture was stirred for 18 h at room temperature, diluted with ether, and quenched with 5% NaHCO3. The ethereal layer was washed with brine, dried over MgSO4, and concentrated by rotary evaporation with a bath temperature maintained at 0 °C. Comparison of NMR signals of this crude material with the commercially available cyclopentane carboxaldehyde confirmed the structure of cyclopentane carboxaldehyde. The crude mixture revealed a 71% NMR yield starting with 2a and a 77% NMR yield starting with 2b.
References:
Hanna, Rana;Daoust, Benoit [Tetrahedron,2011,vol. 67,# 1,p. 92 - 99] Location in patent:experimental part
201230-82-2
1 suppliers
inquiry
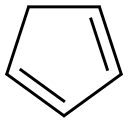
542-92-7
92 suppliers
inquiry

872-53-7
256 suppliers
$9.00/1g
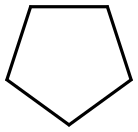
287-92-3
291 suppliers
$22.00/25mL
201230-82-2
1 suppliers
inquiry

542-92-7
92 suppliers
inquiry

872-53-7
256 suppliers
$9.00/1g

4750-17-8
5 suppliers
inquiry

3637-61-4
223 suppliers
$10.00/1g

872-53-7
256 suppliers
$9.00/1g