
Diacetone acrylamide synthesis
- Product Name:Diacetone acrylamide
- CAS Number:2873-97-4
- Molecular formula:C9H15NO2
- Molecular Weight:169.22
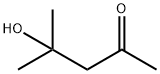
123-42-2
559 suppliers
$20.48/100ml

107-13-1
394 suppliers
$17.00/25ML

2873-97-4
404 suppliers
$5.00/10g
Yield:2873-97-4 70.4%
Reaction Conditions:
with sulfuric acid at 50 - 95; for 2.03111 - 3.27 h;Product distribution / selectivity;Ritter Reaction;
Steps:
1; 2; 3; 4; 5; 7; 8; 9; 10
EXAMPLE 1Use of a Spiral Heat-Exchanger for the First Residence Time UnitA reaction solution with a stoichiometric molar ratio of acrylonitrile (AN) to diacetone alcohol (DiAOH) to H2SO4=1.0:1.0:2.4 had a residence time of 12 s at 90° C. in the microreactor (composed of a mixing module made from Hastelloy and of a heat-exchange module), 100 s at 90° C. in the spiral heat-exchanger, and two hours at 50° C. in the continuous stirred tank.The total yield of diacetoneacrylamide was 78%, based on ACN, the proportions of the final conversion being 74% in the first 12 s, 21% in the following 100 s, and 5% in the remaining 2 h.; EXAMPLES 2-6Execution was analogous to example 1. Table 1 shows starting materials used, residence times, temperatures, and yields:; EXAMPLE 7The reaction led to a final yield of 73.5%, using a stoichiometric ratio of AN to DiaOH to H2SO4=1.0:1.0:2.2 and a residence time distribution, with 90° C. for 12 s in the microreactor, with 65° C. for 960 s in the first continuous stirred tank and with 55° C. for 10 800 s in the second continuous stirred tank.; EXAMPLE 8-10Execution was analogous to example 7. Table 2 shows the starting materials used, residence times, temperatures, and yields:
References:
US2008/306300,2008,A1 Location in patent:Page/Page column 2

107-13-1
394 suppliers
$17.00/25ML
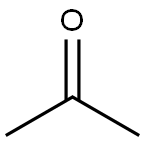
67-64-1
6 suppliers
$19.10/10ml

2873-97-4
404 suppliers
$5.00/10g

107-13-1
394 suppliers
$17.00/25ML

67-64-1
6 suppliers
$19.10/10ml

2873-97-4
404 suppliers
$5.00/10g
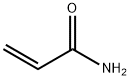
79-06-1
591 suppliers
$17.00/25g