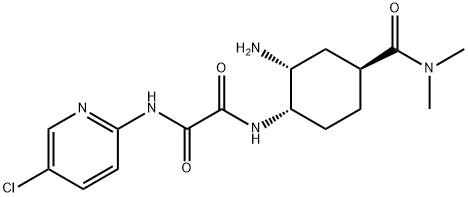
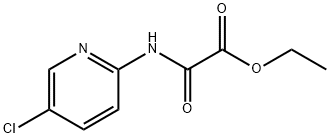
EthanediaMide iMpurity F synthesis
- Product Name:EthanediaMide iMpurity F
- CAS Number:480452-37-7
- Molecular formula:C16H22ClN5O3
- Molecular Weight:367.83
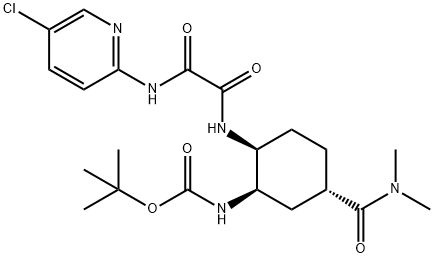
480452-36-6
75 suppliers
inquiry

480452-37-7
54 suppliers
inquiry
Yield: 90%
Reaction Conditions:
with trifluoroacetic acid in dichloromethane at 20; for 4 h;
Steps:
3 Example 3N1-((1S, 2R, 4S) -2-amino-4- (dimethylcarbamoyl) cyclohexyl) -N2- (5-chloropyridin-2-yl) oxalamide 2,2,2-trifluoroacetate 3aOr N1-((1S, 2R, 4S) -2-amino-4- (dimethylcarbamoyl) cyclohexyl) -N2- (5-chloropyridin-2-yl) oxalamide 3Of acid under acidic conditions
Tert-Butyl ((1R, 2S, 5S) -2- (2-((5-chloropyridin-2-yl) amino) -2-oxoacetamido) -5- (dimethylcarbamoyl) cyclohexyl in dichloromethane (30 ml) Carbamate 6 (10.0 g, 21.3 mmol)Was added. To this was added trifluoroacetic acid (19.5 g, 170 mmol) at room temperature. The mixture was then stirred at room temperature for 4 hours.After confirming the completion of the reaction by TLC, the reaction mixture was distilled under rotation to obtain a TFA salt amine 3a.This salt was added to water and treated at room temperature with NaHCO3 (50 ml) to give a solid solution, which was filtered to give the free amine 3 (7.07 g, 90%).
References:
Gapi Bio Company Limited;Hansuk, Chung;Neerasa, jayaprakash JP2019/210273, 2019, A Location in patent:Paragraph 0034; 0035
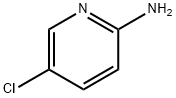
1072-98-6
695 suppliers
$5.00/1g

480452-37-7
54 suppliers
inquiry
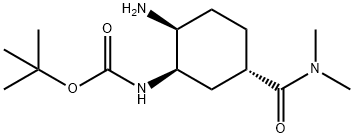
365998-36-3
170 suppliers
$7.00/250mg

480452-37-7
54 suppliers
inquiry
349125-08-2
128 suppliers
$236.47/250mg

480452-37-7
54 suppliers
inquiry