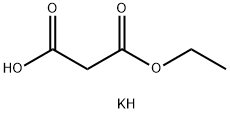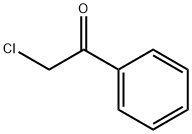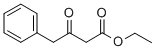
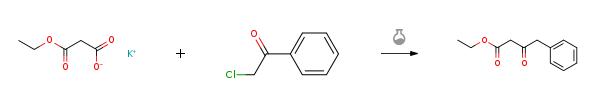
Ethyl 3-oxo-4-phenylbutanoate synthesis
- Product Name:Ethyl 3-oxo-4-phenylbutanoate
- CAS Number:718-08-1
- Molecular formula:C12H14O3
- Molecular Weight:206.24
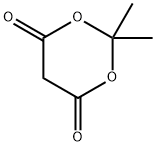
2033-24-1
627 suppliers
$6.00/25g

64-17-5
700 suppliers
$10.00/50g
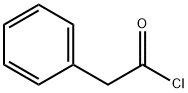
103-80-0
312 suppliers
$13.00/25g

718-08-1
170 suppliers
$100.83/1g
Yield:718-08-1 98.8%
Reaction Conditions:
Stage #1: 2,2-dimethyl-1,3-dioxane-4,6-dione;3?phenylpropanoyl chloridewith pyridine in dichloromethane at 0 - 20;Inert atmosphere;
Stage #2: ethanol for 2.5 h;Inert atmosphere;Reflux;
Steps:
(2) Synthesis of 1t
To the solution of 2,2-dimethyl-1,3-dioxane-4,6-dione (Meldrum’s Acid) (23.75 g, 0.165 mol) and anhydrous pyridine (32.5 mL, 0.4 mol) in CH2Cl2 (100 mL), phenylacetyl chloride (25.50 g, 0.165 mol) was added dropwise under 0 °C. The mixture was stirred at 0 °C for 1 hour and raised to room temperature for another 1 hour. One hundred milliliter 2N aq HCl was added to stop the reaction. The organic phase was separated and the aqueous layer extracted with CH2Cl2. The organic layer was collected, evaporated the solvent to give light yellow solid. After washing by little amount of EtOH, white crystal was obtained and directly refluxed with anhydrous EtOH (250 mL) for 2.5 hours. After concentration under reduced pressure, light yellow oil was obtained which can be used in the next step without purification. Purification by column chromatography with EtOAc-petroleum ether (1:80) yields compound 2 as colorless oil (33.05 g, 98.8%).
References:
Wang, Yiqiong;Zhang, Lingyu;Zhang, Songlin [Synthesis,2022,vol. 54,# 24,p. 5491 - 5499]

91962-64-0
0 suppliers
inquiry

718-08-1
170 suppliers
$100.83/1g
1071-46-1
287 suppliers
$5.00/1g

103-80-0
312 suppliers
$13.00/25g

718-08-1
170 suppliers
$100.83/1g

623-73-4
147 suppliers
$27.30/25ML
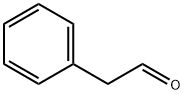
122-78-1
310 suppliers
$10.00/5g

718-08-1
170 suppliers
$100.83/1g