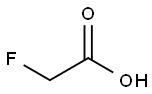
Fluoroacetic acid synthesis
- Product Name:Fluoroacetic acid
- CAS Number:144-49-0
- Molecular formula:C2H3FO2
- Molecular Weight:78.04
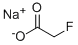
62-74-8
1 suppliers
$50.00/25mg

144-49-0
1 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogenchloride in dichloromethane;water
Steps:
7; 28.1
To a slurry of 1.2 g (12 mmol) of sodium fluoroacetate in 25 mL of CH2CI2 is added, with swirling of the flask, 1 mL (12 mmol) of concentrated HCI (note: this operation must be carried out in an efficient hood; fluoroacetic acid is highly toxic). About 1 teaspoonful of anhydrous magnesium sulfate is added to the flask, and the contents are filtered, rinsing the filter paper with 15 mL of CH2CI2. The combined filtrate and wash are placed under N2 (g), and 1.3 g (8 mmol) of carbonyidiimidazole is added portion-wise to the stirring mixture over 20 min. NMR analysis of an aliquot removed 40 min later indicated that the reaction was nearly complete. After 1 h a teaspoonful of magnesium sulfate is added, and the mixture is allowed to stir overnight. It is filtered and concentrated to remove most of the CH2CI2, leaving 1.6 g of a pale yellow oil. The NMR spectrum indicates the presence of CH2CI2, fluoroacetic acid, imidazole, and fluoroacetyl imidazole : 1H NMR (CDC13) 8 8.26 (s, 1 H), 7.53 (s, 1 H), 7.15 (s, 1 H), 5.40 (d, J= 47 Hz, 2 H). Integration reveals the oil to be 28% by weight fluoroacetyl imidazole (0.45 g, 3.5 mmol, 44%). The oil is diluted with CH2CI2 to make a solution that is 0.2 M fluoroacetyl imidazole.Scheme 8 Step 1. Preparation of fluoroacetyl imidazole (29). To a slurry of 1.2 g (12 mmol) of sodium fluoroacetate in 25 mL of CH2CI2 is added, with swirling of the flask, 1 mL (12 mmol) of concentrated HCI (note: this operation must be carried out in an efficient hood; fluoroacetic acid is highly toxic). About 1 teaspoonful of anhydrous magnesium sulfate is added to the flask, and the contents are filtered, rinsing the filter paper with 15 mL of CH2CI2. The combined filtrate and wash are placed under nitrogen, and 1.3 g (8 mmol) of carbonyidiimidazole is added portion-wise to the stirring mixture over 20 min. NMR analysis of an aliquot removed 40 min later indicates nearly complete reaction. After 1 h a teaspoonful of magnesium sulfate is added, and the mixture is allowed to stir overnight. It is filtered and concentrated to remove most of the CH2CI2, leaving 1.6 g of a pale yellow oil. The NMR spectrum indicates the presence of CH2CI2, fluoroacetic acid, imidazole, and fluoroacetyl imidazole 29 : 1H NMR (CI3) 6 8.26 (s, 1 H), 7.53 (s, 1 H), 7.15 (s, 1 H), 5.40 (d, J = 47 Hz, 2 H). Integration reveals the oil to be 28% by weight fluoroacetyl imidazole 29 (0.45 g, 3.5 mmol, 44%). The oil is diluted with CH2CI2 to make a solution that is 0.2 M in 29.
References:
ELAN PHARMACEUTICALS, INC. WO2005/70407, 2005, A1 Location in patent:Page/Page column 148; 172
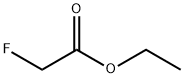
459-72-3
201 suppliers
$10.00/1g

144-49-0
1 suppliers
inquiry
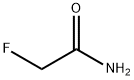
640-19-7
4 suppliers
$60.00/500mg

144-49-0
1 suppliers
inquiry

1544-46-3
15 suppliers
inquiry

144-49-0
1 suppliers
inquiry
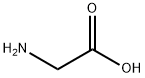
56-40-6
1197 suppliers
$5.00/25g

144-49-0
1 suppliers
inquiry