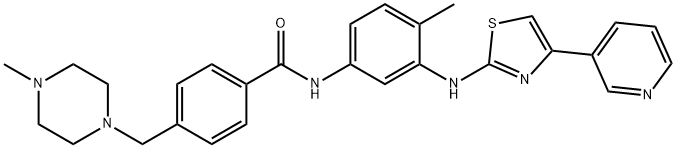
Masitinib synthesis
- Product Name:Masitinib
- CAS Number:790299-79-5
- Molecular formula:C28H30N6OS
- Molecular Weight:498.64
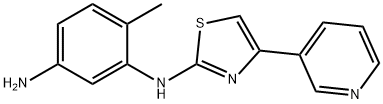
660837-08-1
38 suppliers
inquiry
![METHYL 4-[(4-METHYLPIPERAZIN-1-YL)METHYL]BENZOATE](/CAS/GIF/314268-40-1.gif)
314268-40-1
79 suppliers
$90.00/500mg

790299-79-5
235 suppliers
$29.00/10mg
Yield:790299-79-5 78%
Reaction Conditions:
Stage #1: N1-[4-(pyridin-3-yl)-1,3-thiazol-2-yl]-6-methyl-1,3-phenylenediaminewith trimethylaluminum in hexane;dichloromethane at 5 - 15; for 2 h;
Stage #2: 4-((4-methylpiperazin-1-yl)methyl)-benzoic acid methyl ester in hexane;dichloromethane at 20; for 21.1667 h;Heating / reflux;
Stage #3: with sodium hydroxide;water in hexane;dichloromethane at 20; for 3 h;Product distribution / selectivity;
Steps:
4.A
Method A In a reactor and under low nitrogen pressure, add 4-Methyl-N3-(4-pyridin-3-yl-thiazol- 2-yl)-benzene-l,3-diamine (95 g, 336.45 mmol), dichloromethane (2 L). To this
References:
WO2008/98949,2008,A2 Location in patent:Page/Page column 20-21

660837-08-1
38 suppliers
inquiry

148077-69-4
75 suppliers
$680.00/250mg

790299-79-5
235 suppliers
$29.00/10mg

660837-08-1
38 suppliers
inquiry

106261-48-7
192 suppliers
$17.67/5gm:

790299-79-5
235 suppliers
$29.00/10mg
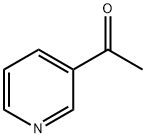
350-03-8
639 suppliers
$10.00/5g

790299-79-5
235 suppliers
$29.00/10mg

660838-06-2
1 suppliers
inquiry

790299-79-5
235 suppliers
$29.00/10mg