
Methyl 5-nitroisophthalate synthesis
- Product Name:Methyl 5-nitroisophthalate
- CAS Number:1955-46-0
- Molecular formula:C9H7NO6
- Molecular Weight:225.15

13290-96-5
387 suppliers
$8.00/25g

1955-46-0
246 suppliers
$9.00/5g
Yield:1955-46-0 75%
Reaction Conditions:
with water;sodium hydroxide in methanol for 2 h;Reflux;
Steps:
2 Example 2.Synthesis of monomethyl 5-nitroisophthalate
To a 250 mL four-necked flask equipped with a stirrer, a condenser, and a thermometer, 2.5 g of dimethyl 5-nitroisophthalate and 38.5 mL of methanol were added and dissolved by heating and stirring,Then in 20min0.42 g of sodium hydroxide dissolved in 5 g of water was added dropwise, and the reaction was heated to reflux for 2 h. The reaction mixture was followed by TLC to disappear at the point of starting material, developing solvent (petroleum ether: CH2Cl2 = 5: 1);Take a small amount of acidification,Using 5-nitro-isophthalic acidandMonomethyl 5-nitro-isophthalate as a control,TLC shows whether there is product formation and the presence of 5-nitro-isophthalic acid, developing solvent (CH2Cl2: CH3OH = 5: 1). After completion of the reaction, methanol was distilled off, and 26 mL of water was added thereto. The filtrate was filtered while hot, and the filtrate was acidified to pH 1 with concentrated hydrochloric acid. The crystals were cooled and washed with water, and the filter cake was dried to give 5-nitroisophthalic acid monomethyl Ester was obtained in a yield of 75%
References:
Zunyi Medical University;Zhao, Changkuo;Wang, Xianheng;Ma, Jiahui;Yang, Fuhong CN105254521, 2016, A Location in patent:Paragraph 0026
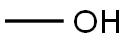
67-56-1
733 suppliers
$7.29/5ml-f

618-88-2
480 suppliers
$13.00/25g

1955-46-0
246 suppliers
$9.00/5g

618-88-2
480 suppliers
$13.00/25g

1955-46-0
246 suppliers
$9.00/5g