OlMesartan MedoxoMil Ethyl Methyl Analog synthesis
- Product Name:OlMesartan MedoxoMil Ethyl Methyl Analog
- CAS Number:1378863-74-1
- Molecular formula:C30H32N6O6
- Molecular Weight:572.61
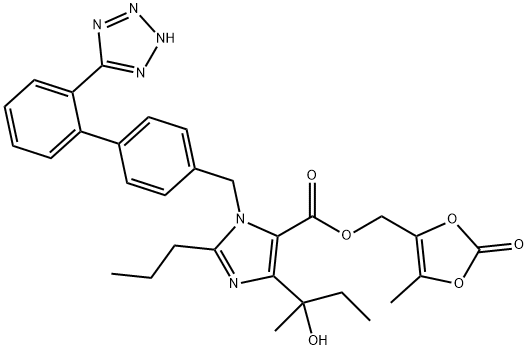
![(5-methyl-2-oxo-1,3-dioxol-4-yl)methyl 4-(2-hydroxybutan-2-yl)-2-propyl-1-((2'-(1-trityl-1H-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)-1H-imidazole-5-carboxylate](/CAS/20180703/GIF/1378863-72-9.gif)
1378863-72-9
5 suppliers
inquiry

1378863-74-1
25 suppliers
$650.00/10mg
Yield:-
Reaction Conditions:
with water;acetic acid at 50; for 4 h;
Steps:
6
(5-Methyl-2-oxo- 1 ,3-dioxol-4-ylmethyl) 4-( 1 -hydroxy- 1 -methylpropyl)-2-propyl- 1 -[2 '-( 1H- tetrazol-5-yl)biphenyl-4-yl-methyl]imidazole-5-carboxylate of formula VI (5-Methyl-2-oxo- 1 ,3-dioxol-4-yl)methyl 4-( 1 -hydroxy- 1 -methylpropyl)-2-propyl- 1 -((2'-( 1 - trityl-lH-tetrazol-5-yl)biphenyl-4-yl)methyl)-lH-imidazole-5-carboxylate of formula 6; 2,1 g were dissolved in acetic acid (10 ml) and after addition of water (5 ml) the mixture was heated to 50 °C for 4 h. After aspiration of trityl alcohol, evaporation and concentration crystallization from ethyl acetate was performed. 1.14 g of the product were obtained in the form of a white, crystalline substance with the content of 94.4% by weight as determined using the HPLC method in accordance with Example 1. NMR spectrum (CDC13): 0.85 t, 3 H, J= 7.5; 0.90 t, 3 H, J= 7.4; 1,55 s, 3 H; 1.65-1.70 m, 2 H; 1.80-1.90 m, 1 H; 1.95-2.05 m, 1 H; 2.20 s, 3 H; 2.55 t, 2 H, J = 7.5; 4.95 s, 2 H; 5.35 d, 1 H, J = 17.1 ; 5.45 d, 1 H, J = 17.1 ; 6.80 d, 2 H, J = 8.2; 7.10 d, 2 H, J= 8.2; 7.45 t, 1 H, J = 6.9; 7.50-7.55 m, 1 H; 7.55-7.60 m, 1 H; 7.80 d, 1 H, J = 7.7 13C NMR spectrum (CDC13) 5.53, 9.41, 13.75, 21.17, 26.31, 29.10, 34.55, 49.10, 50.16, 53.84, 62.66, 73.41, 116.57, 122.79, 125.24, 128.24, 129.51, 130.71, 131.01, 131.37, 133.22, 135.25, 136.48, 138.72, 140.56, 140.91, 152.49, 152.88, 154.99, 160.26, 160.74.HR-MS: pro C30H33N6O6 [M+H]+, calculated 573.2456 m/z, found 573.2456 m/z.
References:
WO2012/69025,2012,A1 Location in patent:Page/Page column 12
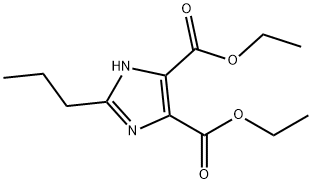
144689-94-1
271 suppliers
$8.00/5g

1378863-74-1
25 suppliers
$650.00/10mg

172875-53-5
67 suppliers
inquiry

1378863-74-1
25 suppliers
$650.00/10mg